Method for rapidly preparing intercalation-type vermiculite by dry method
A vermiculite, fast technology, applied in chemical instruments and methods, inorganic chemistry, non-metallic elements, etc., can solve the problems of long intercalation reaction time, low efficiency, unfavorable industrial production, etc., to improve the intercalation reaction efficiency, simplify the Craftsmanship, the effect of reduced time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The vermiculite of Shijiazhuang Dongping Mining and Building Materials Factory was pulverized through a jet mill and passed through a 100-mesh sieve to obtain the raw material vermiculite VMT.
[0016] The chemical composition is: SiO 2 : 41.2%, AL 2 o 3 : 12.68%, MgO: 24.22%, CaO: 0.96%, FeO: 1.54%, Fe 2 o 3 : 4.06%, TiO 2 : 1.33%, K 2 O: 5.97%, P 2 o 5 : 0.06%, H 2 O: 3%, Na 2 O: 1.6%, MnO: 0.043%, TiO: 5.097%, loss on ignition 6.71%.
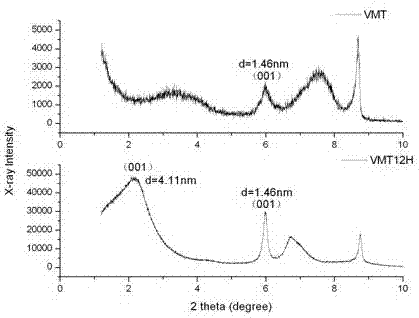
[0017] Put 40 grams of VMT sample and 2 times of CEC dodecyltrimethylammonium bromide into the HAAKE torque rheometer, mix and stir at 120°C for 30min at a speed of 120rpm, take out the sample after centrifugation and filtration, Repeatedly washed 5 to 7 times with deionized water, after Ag + No white precipitate (AgBr) was detected. Finally, the sample obtained by centrifugation and filtration was dried and ground into powder to obtain sample VMT12H. X-ray powder diffraction (XRD) tests were performed on VMT and VMT12H res...
Embodiment 2
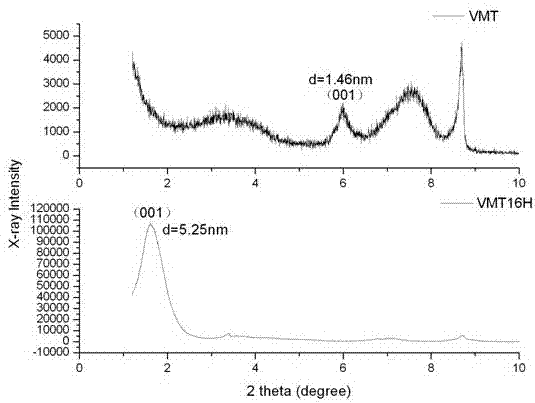
[0020] Put 40 grams of VMT sample and cetyltrimethylammonium bromide with 2 times CEC into the HAAKE torque rheometer, mix and stir at 120°C for 30min at a speed of 120rpm, take out the sample after centrifugation and filtration, Repeatedly washed 5 to 7 times with deionized water, after Ag + No white precipitate (AgBr) was detected. Finally, the sample obtained by centrifugation and filtration was dried and ground into powder to obtain sample VMT16H. X-ray powder diffraction (XRD) tests were performed on VMT and VMT12H respectively, and the corresponding patterns are as follows image 3 shown.
[0021] image 3 It is the XRD comparison pattern of samples VMT and VMT16H, the (001) plane diffraction peak of VMT in the figure is d The value, that is, the (001) interplanar spacing is 1.46nm. VMT16H d The diffraction peak with a value of 1.46nm disappeared, and a new diffraction peak at 5.25nm appeared at the same time. It shows that after dry intercalation, the intercalatio...
Embodiment 3
[0023] Put 40 grams of VMT sample and octadecyltrimethylammonium bromide with 2 times CEC into the HAAKE torque rheometer, mix and stir at 120°C for 30min at a speed of 120rpm, take out the sample after centrifugation and filtration, Repeatedly washed 5 to 7 times with deionized water, after Ag + No white precipitate (AgBr) was detected. Finally, the sample obtained by centrifugation and filtration was dried and ground into powder to obtain sample VMT18H. X-ray powder diffraction (XRD) tests were performed on VMT and VMT18H respectively, and the corresponding patterns are as follows Figure 4 shown.
[0024] Figure 4 It is the XRD comparison pattern of samples VMT and VMT18H, the (001) plane diffraction peak of VMT in the figure is d The value, that is, the (001) interplanar spacing is 1.46nm. VMT18H d The diffraction peak with a value of 1.46nm disappeared, and a new diffraction peak at 4.58nm appeared at the same time. It shows that after dry intercalation, the interc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com