Plinabulin compound polycrystalline type and preparation method thereof
A technology of crystal form and β crystal form, which is applied in the field of polymorphic forms of dehydrophenylahistine compounds and their preparation, can solve problems such as restricting application, and achieves clear conformation, high purity and method reproducibility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)methylene)piperazine-2,5-dione α crystal form
[0038] Its specific preparation process technology includes the following steps:
[0039] 1) Preparation of ethyl 5-(tert-butyl)oxazole-4-carboxylate
[0040] Add 90g (796mmol) ethyl isocyanoacetate to 1000mL tetrahydrofuran, slowly dropwise add 145g (955mmol) DBU, then dropwise add 178g (955mmol) trimethylacetic anhydride, and stir the reaction at room temperature for 48h after dropping. After the reaction was completed, it was concentrated under reduced pressure. For extraction, add an appropriate amount of 1500mL of dichloromethane, wash with 800mL of 10% sodium carbonate, 800mL of 10% citric acid, and 800mL of saturated brine, and back-extract the aqueous phase twice with 1000mL of dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, filtered with suction after half an hour, and concentrated under reduced pressur...
Embodiment 2
[0055] Preparation of β Crystal Form of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)methylene)piperazine-2,5-dione
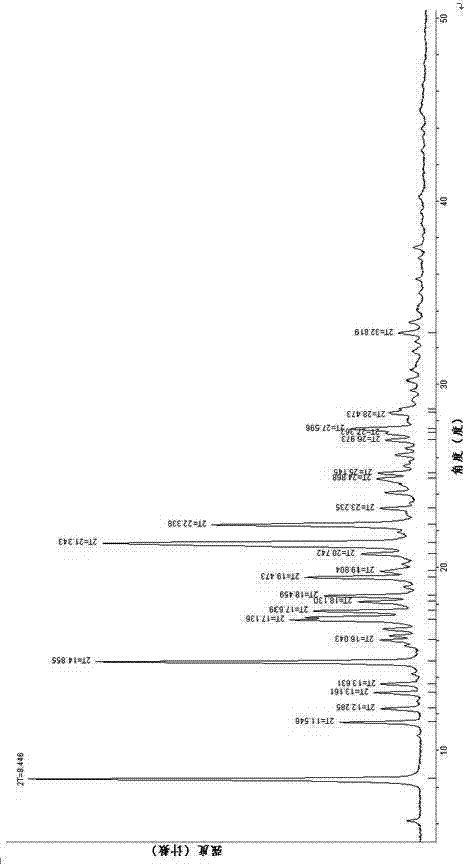
[0056] The specific preparation process includes the following steps: Weigh the (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)methylene)piperazine-2 , 5-diketone (200mg, 0.59mmol), using a mixed solution of 20mL methanol and 0.1mL water as a solvent, dissolved at 70°C, filtered into a crystallization dish, covered the bottle mouth of the crystallization dish with plastic wrap, and placed on the plastic wrap Prick 16 holes with a capillary tube with an outer diameter of 0.5 mm, and place it in the dark for evaporation at room temperature. After 72 hours, crystals of the β crystal form precipitated, filtered, and dried to obtain 148 mg of a cubic solid, with a yield of 74%. The obtained β crystal form was tested by X-ray powder diffraction, and the characteristic absorption peaks of 2θ diffraction angles were 7.365°, 7.670°, 8.097°, 9.069°, 1...
Embodiment 3
[0062] Preparation of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)methylene)piperazine-2,5-dione γ crystal form
[0063] The specific preparation process includes the following steps: Weigh the (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)methylene)piperazine-2 , 5-diketone (200mg, 0.59mmol), using a mixed solution of 20mL methanol and 0.8mL water as a solvent, dissolved at 68°C, filtered into a crystallization dish, added seed crystals, covered the mouth of the crystallization dish with plastic wrap, Prick 16 holes on the plastic wrap with a capillary tube with an outer diameter of 0.5 mm, and place it in the dark for evaporation at room temperature. After 72 hours, crystals of the γ crystal form precipitated, filtered, and dried to obtain 98 mg of needle-columnar solids with a yield of 49%. The obtained γ crystal form was tested by X-ray powder diffraction, and the characteristic absorption peaks of 2θ diffraction angles were 7.918°, 9.168°, 9.905°, 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap