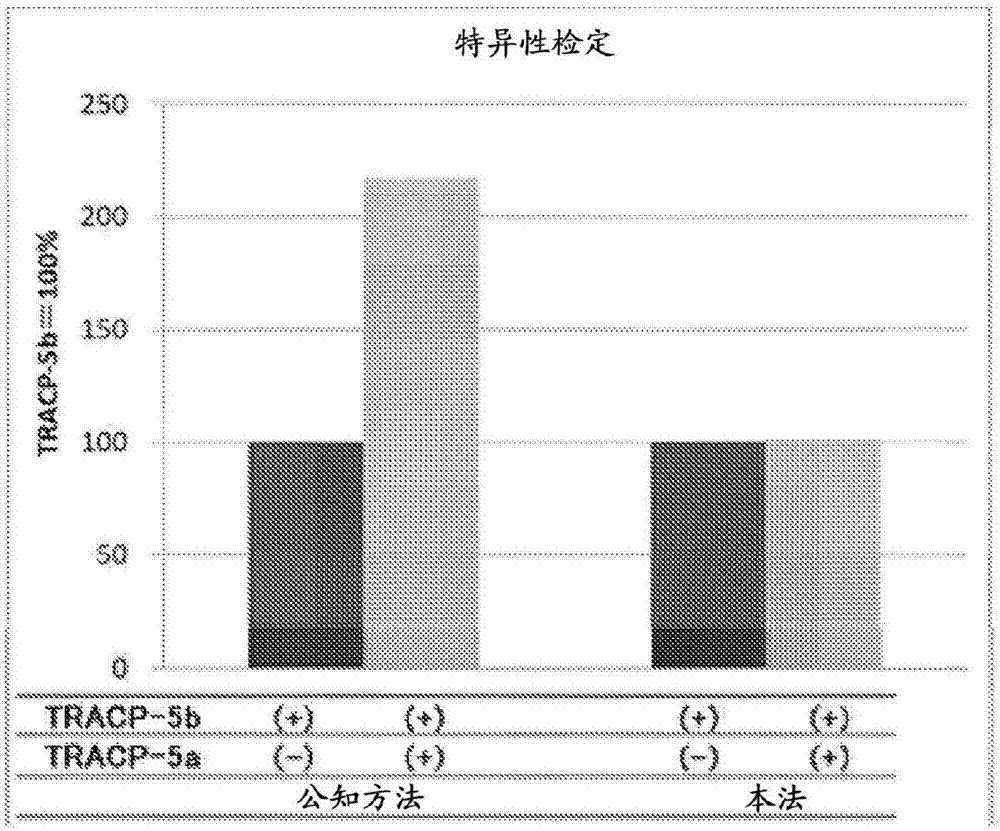
Protein assay method specific to tracp-5b (tartrate resistant acid phosphatase 5b)
一种tracp-5b、NITEBP-01866的技术,应用在抗酶免疫球蛋白、抗动物/人类的免疫球蛋白、特定肽等方向,达到辅助诊断的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0089] The monoclonal antibody of the present invention can be obtained by using recombinant human TRACP-5b as an immunogen. In the examples described later in this specification, the hosts for purification from genetically recombinant silkworms are not limited as long as they are cultured cells, Escherichia coli , etc., as long as they can express human TRACP-5b.
[0090] The monoclonal antibody of the present invention is produced, for example, by a hybridoma obtained by immunizing an animal with purified human TRACP-5b as an immunogen, and fusing anti-human TRACP-5b antibody-producing cells produced by the animal with myeloma cells.
[0091] The above-mentioned hybridomas can be obtained by the following method. That is, by mixing the human TRACP-5b obtained as described above using Freund's complete and incomplete adjuvants, aluminum hydroxide adjuvants, pertussis adjuvants, etc., to prepare an adjuvant solution for sensitization, fraction Animals such as mice and rats ar...
Embodiment 1
[0123] (1) Selection and preparation of antigens for monoclonal antibody production
[0124] As an antigen for preparing anti-human TRACP monoclonal antibody, recombinant TRACP-5b produced in the silk gland of genetically recombinant silkworm was prepared. The preparation method of the recombinant TRACP-5b was prepared by the method described in Japanese Patent No. 5177431.
[0125] The silk gland was extracted from the genetically recombinant silkworm containing human recombinant TRACP-5b to obtain 300 g of silk gland. The silk glands were suspended in 5600 mL of buffer solution (50 mM Tris-HCl, pH 7.5), and homogenized with a rotor stator type homogenizer. Thereafter, centrifuge at 10,000 rpm for 20 minutes and apply the supernatant to a CM-Sepharose column (GE Healthcare), the adsorbed protein was eluted with a linear concentration gradient (0-1.0 M NaCl) of the above-mentioned Tris buffer containing NaCl. The tartrate-resistant acid phosphatase activity was measured us...
Embodiment 2
[0179] Epitope analysis of TrK-126 and TrK-127
[0180] (1) Epitope analysis of TrK-126 and TrK-127 by Replitope
[0181] Based on the above results, epitope analysis of TrK-126 and TrK-127 was carried out by the luminescence method.
[0182] Specifically, the above-mentioned peptide slides were blocked at room temperature for 60 minutes using SuperBlock (PIERCE). Thereafter, as the primary antibody, TrK-126 or TrK-127 was reacted at a concentration of 1 μg / mL at room temperature for 60 minutes. After washing with PBS-T 3 times, the secondary antibody attached to Amersham ECL Prime (GE Healthcare) was added and reacted at room temperature for 60 minutes. After washing 3 times with PBS-T again, add the detection reagent attached to the kit. After a certain period of incubation, the degree of luminescence of the plaques was confirmed by ImageQuant LAS4000 (GE Healthcare).
[0183] As a result of the analysis, neither TrK-126 nor TrK-127 showed reactivity with each sequence p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com