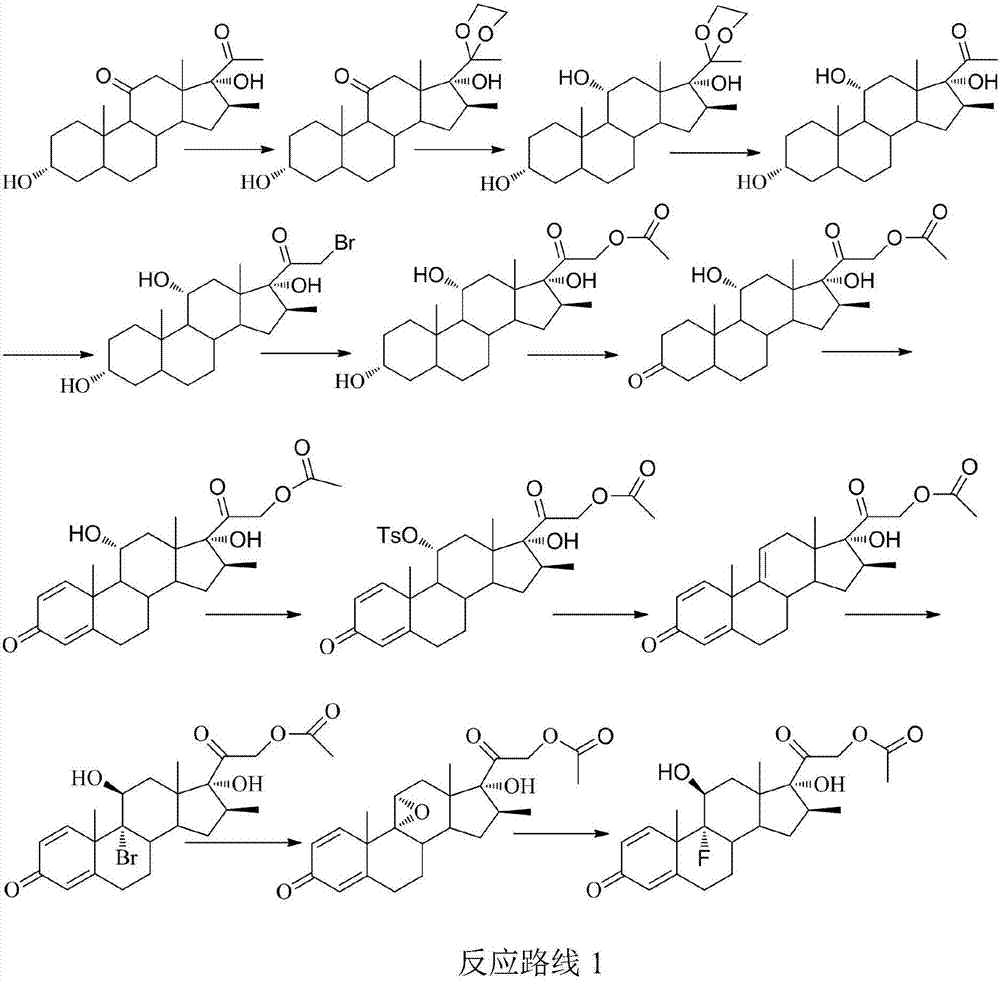
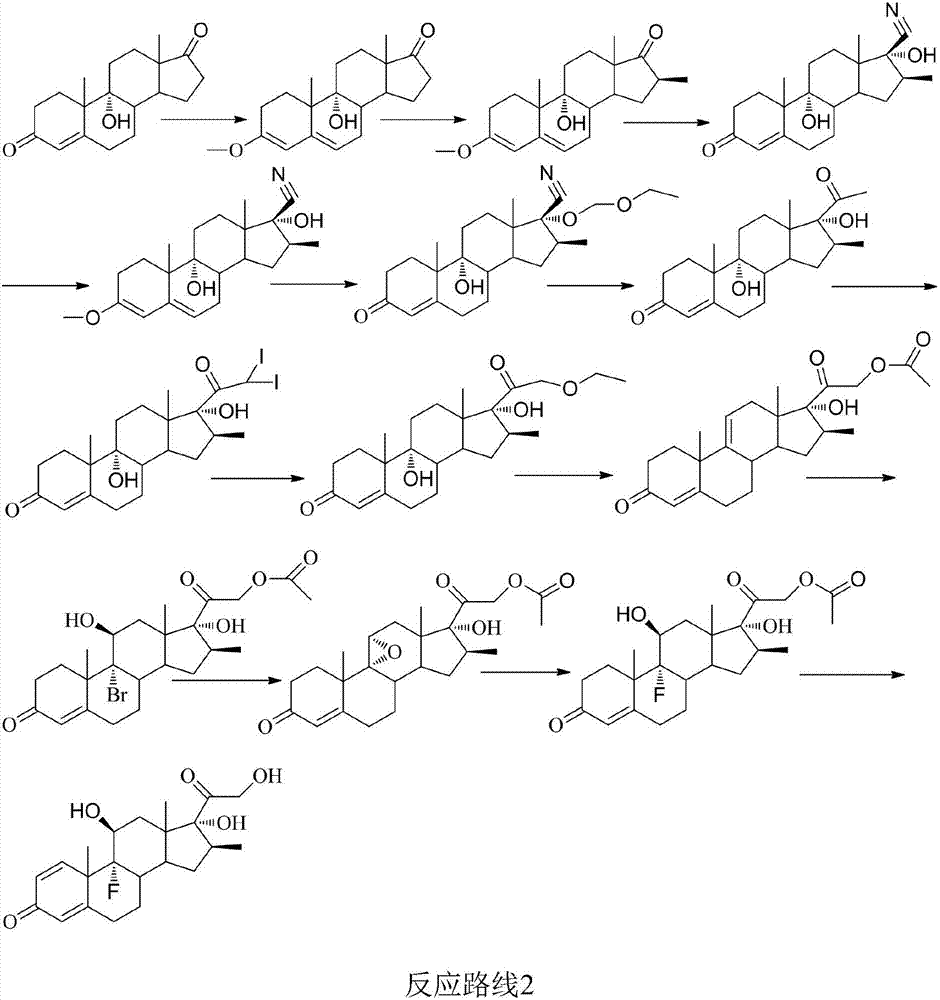
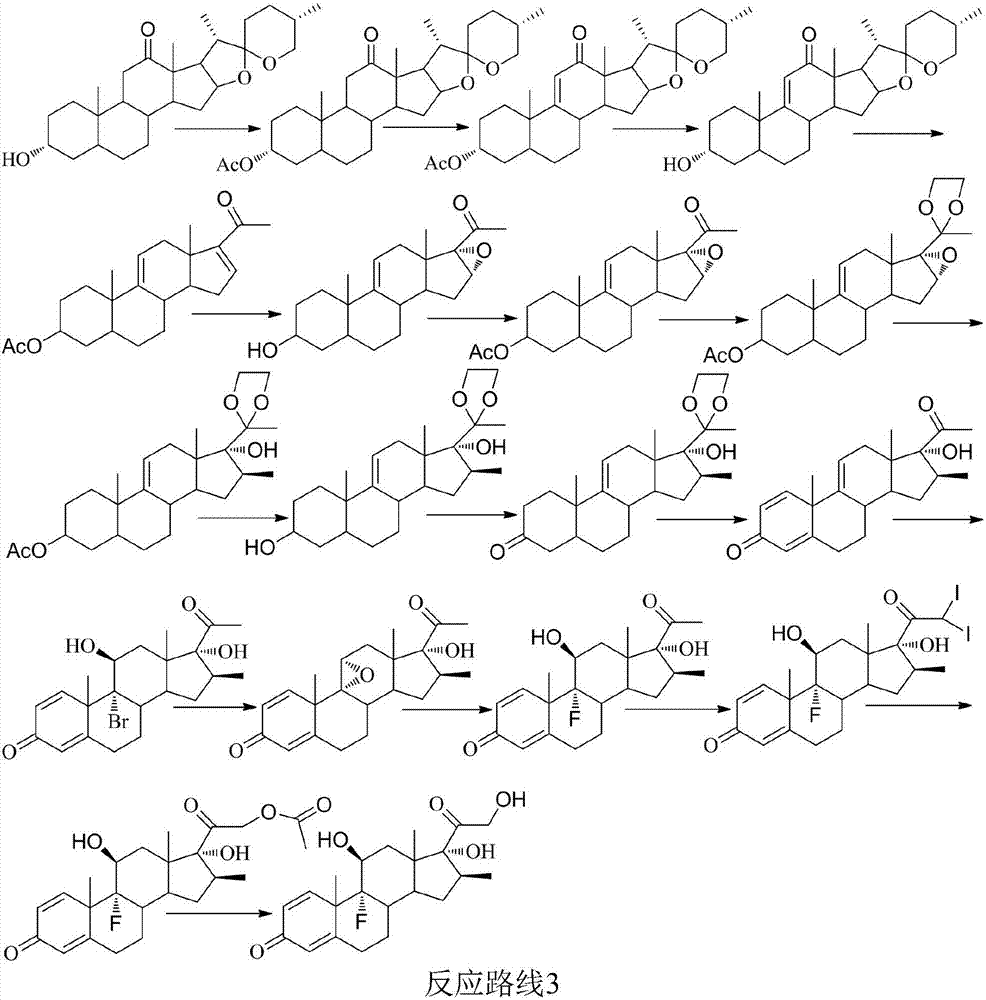
Betamethasone preparation method
A technology of betamethasone and its compounds, which is applied in the field of preparation of betamethasone, can solve problems such as unfavorable industrial production, lower yield, difficult post-processing, etc., and achieve the effect of promoting technological innovation and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of thiophenylchloride: under nitrogen protection, add 115.5mL thiophenol, 530mL n-pentane, and 3.3mL triethylamine into a 1000mL flask, stir, cool down to -5~0°C, add 104.5mL sulfuryl chloride dropwise, drop During the addition process, a white solid was formed, and then the solid gradually dissolved. After the dripping, the ice bath was removed, and the temperature was slowly raised to 25°C, and the reaction was kept for 1.5 hours. When the color of the reaction solution changed from yellow to deep red, the reaction was stopped. Concentrate under reduced pressure at 40°C until there is no more dripping, then raise the temperature to 50°C and concentrate in vacuum for 1.5 hours to obtain 169g of phenylthiochloride (dark red oily substance), which is kept in the refrigerator for later use. Preparation of triethylamine acetate:
[0066] Under the protection of nitrogen, add 41.3g of triethylamine into a 250ml four-neck bottle, stir well, cool down to 10°C, add...
Embodiment 2
[0073] Preparation of triethylamine acetate:
[0074] Under the protection of nitrogen, add 12.53g of triethylamine into a 250ml four-neck bottle, stir evenly, cool down to 10°C, add 7.44g of glacial acetic acid dropwise, the temperature control during the dropwise addition should not exceed 25°C, and stir at room temperature for 1 hour after dropping , to get triethylamine acetate, set aside.
[0075] Preparation of Compound B:
[0076] Under the protection of nitrogen, add 40g of compound A and 400mL of dichloromethane into a 2000ml four-neck flask, stir evenly, add dropwise the solution of 19.9g of triethylamine acetate and 101mL of dichloromethane prepared above, and cool down to -70°C , dropwise the solution of 16.79g phenylthiochloride (prepared by the method for preparing phenylthiochloride in Example 1) dissolved in 16.7mL of dichloromethane, dropwise process temperature control-70 ℃, drop time is 1 hour, reaction After 1 hour, TLC (PE:EA=3:1) showed that after the r...
Embodiment 3
[0081] Preparation of triethylamine citrate:
[0082] Under the protection of nitrogen, add 60mL of n-hexane and 34.1g of triethylamine into a 250ml four-necked bottle, stir evenly, cool down to 10°C, add 21.6g of citric acid in batches, and control the temperature during the addition to no more than 25°C. Stir at room temperature for 1 hour, and concentrate the solvent to dryness to obtain triethylamine citrate, which is set aside.
[0083] Preparation of Compound B:
[0084] Under the protection of nitrogen, add 54.5g of compound E and 500mL of dichloromethane into a 2000ml four-necked bottle, stir well, add 55.5g of triethylamine citrate prepared above, cool down to -72°C, and dropwise add 45.6g of phenylsulfide Chlorine (prepared by the method for the preparation of phenylthiochloride in Example 1) was dissolved in a solution of 456mL of dichloromethane, the temperature of the dropwise addition process was controlled at -72°C, the dropwise addition time was 3 hours, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com