Synthesis method of organic compound of diamine monoselenide
A technology of organic compounds and diamine-based monos is applied in the field of synthesizing diamine-based monoselenide organic compounds, can solve problems such as few reports on synthetic methods, and achieve the effects of high scientific research value, low cost of raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention is a simple and efficient synthesis method of diamine monoselenide. The synthesis steps are as follows:
[0022] 1) Use ethyl ethanolamine (BOC) 2 O protection.
[0023] 2) Acylation of its hydroxyl group.
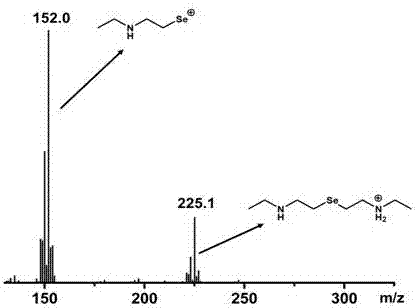
[0024] 3) Reaction with sodium selenium hydride to obtain a monoselenide bridging compound, and then remove the BOC protecting group with trifluoroacetic acid.
[0025] 4) extracting with an organic solvent to obtain diamine monoselenide.
[0026] Add di-tert-butyl dicarbonate dropwise to ethyl ethanol, the dosage ratio is 1:1.5, this method has sufficient reaction and high yield. Above-mentioned step 3) is anaerobic operation under ice bath condition, and reaction is more fully, and by-product is few. After removing the BOC protecting group with trifluoroacetic acid, the product was dissolved in an alkaline solution to form a salt.
[0027] The solvents used in the present invention are all organic solvents, and dichloromethane, ethyl acetate, m...
Embodiment 1
[0030] To a 250ml round bottom flask was added 4.5g ethylethanolamine (0.05mol) and dissolved in 50ml dichloromethane.
[0031] At 30° C., 16.37 g of di-tert-butyl dicarbonate (0.75 mol) dissolved in 50 ml of dichloromethane was slowly added dropwise into the round bottom flask, and the dropwise addition continued for 1 hour.
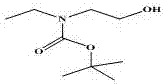
[0032] After the dropwise addition was complete, it was stirred at room temperature for 12 hours. After the reaction was completed, the dichloromethane solution was extracted three times each with 300ml of 0.1mol hydrochloric acid, 300ml of 5% sodium bicarbonate solution, and 300ml of saturated sodium chloride solution. The organic phase was dried with anhydrous sodium sulfate, then filtered, and the organic phase was spin-dried under reduced pressure with a rotary evaporator to obtain a transparent liquid compound 1 (9.4 g, yield 99.3%).
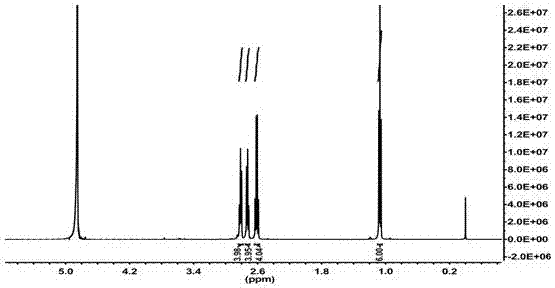
[0033] The structural formula of compound 1 is as follows:
[0034]
[0035] δ H (500 MHz; CDCl 3 ) 1.11 (...
Embodiment 2
[0055] To a 250ml round bottom flask was added 4.5g ethylethanolamine (0.05mol) and dissolved in 50ml dichloromethane.
[0056] Under the condition of 30° C., 10.91 g of di-tert-butyl dicarbonate (0.05 mol) dissolved in 50 ml of dichloromethane was slowly added dropwise into the round bottom flask, and the dropwise addition continued for 1 hour.
[0057] After the dropwise addition was complete, it was stirred at room temperature for 12 hours. After the reaction was completed, the dichloromethane solution was extracted three times each with 300ml of 0.1mol hydrochloric acid, 300ml of 5% sodium bicarbonate solution, and 300ml of saturated sodium chloride solution. The organic phase was dried over anhydrous sodium sulfate,
[0058] Into a 250ml round bottom flask was added 4g of compound 1 (21.2mmol) dissolved in 50ml of dry dichloromethane, and then 8.8ml of triethylamine (63.5mmol) was added to the solution.
[0059] 4.04 g of p-toluenesulfonyl chloride (21.2 mmol) dissolved...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com