Method for increasing expression quantity of Fab antibody
An expression level and antibody technology, applied in the field of genetic engineering, can solve the problems of losing industrial application prospects, achieve good practical value and scientific research significance, and improve the effect of Fab expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The method for increasing the expression of Fab antibody provided in this application mainly depends on the transformation of Escherichia coli. In this example, the original host cell strain Escherichia coli W3110 (W3110(F-l-rph-1 INV(rrnD,rrnE)ilvG) was taken as an example. The preparation process is briefly described as follows.
[0047] (1) Construct recombinant plasmids pKO3-pepN, pKO3-DegQ, pKO3-dcp and pKO3-pepP respectively
[0048] The artificially synthesized pepN, DegQ, dcp, and pepP integration cassettes (recombined gene sequences) were subjected to SalI and NotI double enzyme digestion, and the pKO3 plasmid was subjected to SalI and NotI double enzyme digestion, and then the digested products were ligated, respectively. Construction of recombinant plasmids pKO3-pepN, pKO3-DegQ, pKO3-dcp and pKO3-pepP;
[0049] It needs to be explained that there are three main reasons for choosing the pKO3 plasmid: first, the pKO3 plasmid uses the replication origin (RepA)...
Embodiment 2
[0090] According to the current routine transgenic operation method, the recombinant plasmid pVEGF-Fab was constructed for the expression of Fab antibody.
[0091] Since it is necessary to use pETDuet-1 with two independent promoters as the expression vector for double-gene co-expression, it is necessary to properly optimize the coding gene of Fab. The principles of gene optimization are as follows:
[0092] An independent leader peptide sequence from the periplasm of Gram-negative bacteria is added to the N-terminus of the light chain and the heavy chain Fd of the Fab coding gene, specifically:
[0093]The amino acid sequence of OmpA was added before the light chain: KKTAIAIAVALAGFATVAQA, after codon optimization according to the E. coli prokaryotic expression system, the full-length light chain DNA was synthesized by the solid-phase phosphoramidite method, and inserted into after the first promoter;
[0094] The OmpA amino acid sequence was added before the Fd segment of th...
Embodiment 3
[0097] Pick the transformed positive single colony in Example 2 and add it to 5 mL of 2×PY (1% plant peptone, 0.5% yeast extract, 0.5% NaCl) culture solution containing 10 μg / mL tetracycline, at 37°C, 250rpm Incubate overnight with shaking;
[0098] Inoculate 100 μL of the overnight culture into 200 mL of SM6E medium supplemented with tetracycline (10 μg / mL), culture overnight at 30°C with shaking at 250 rpm; repeat this step once until the culture reaches OD 600 About 2, and then directly used or long-term storage for future use (the long-term storage procedure is: centrifuge, collect the cells, then resuspend the cells in 100mL of SM6E, add glycerol at a final concentration of 12.5%, store at -80°C)
[0099] Take 2 mL of the culture, add it to 200 mL of SM6E medium containing 10 μg / mL tetracycline, and culture it at 30°C with shaking at 250 rpm until OD 600 about 2.
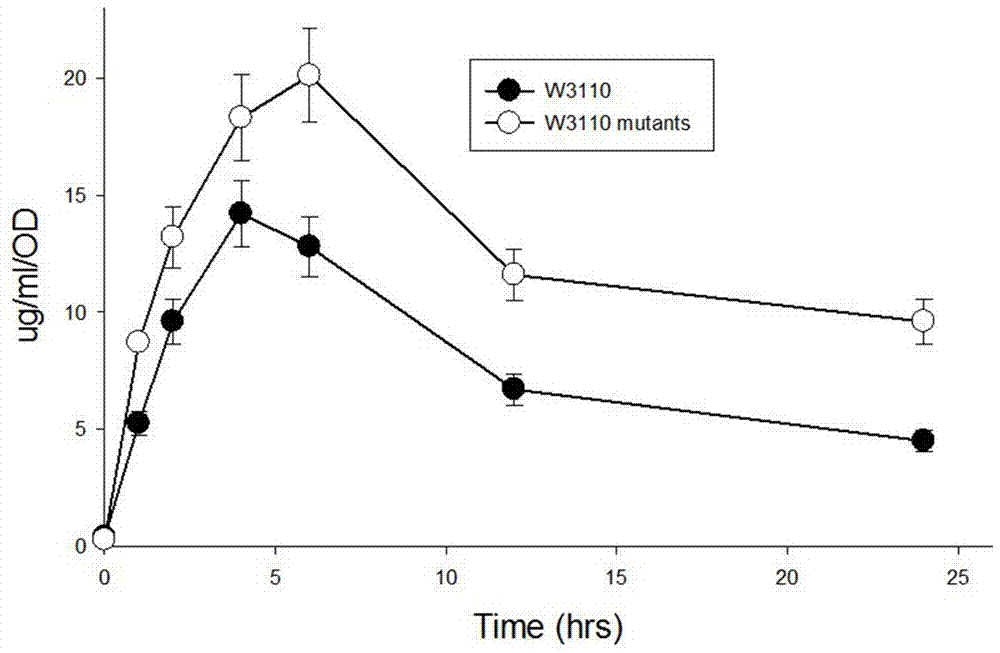
[0100] Add IPTG to a final concentration of 200 μM to induce recombinant protein production. At 1, 2, 4, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com