Combined reactor and method of preparing pyrazolyl benzaldehyde acetal p-phenylene diamine bis-schiff base by using combined reactor
A combined reactor, double Schiff base technology, applied in chemical instruments and methods, chemical/physical/physical-chemical reactors, instruments, etc., can solve problems such as insufficient reaction, achieve rapid cooling, rapid air cooling, and improve The effect of efficiency and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A combined reactor, comprising a magnetic stirrer capable of precise temperature control and a reactor placed in the magnetic stirrer, the reactor is a three-necked flask, the flask is equipped with a dropping funnel, the dropping funnel Installed with slow flow connector;
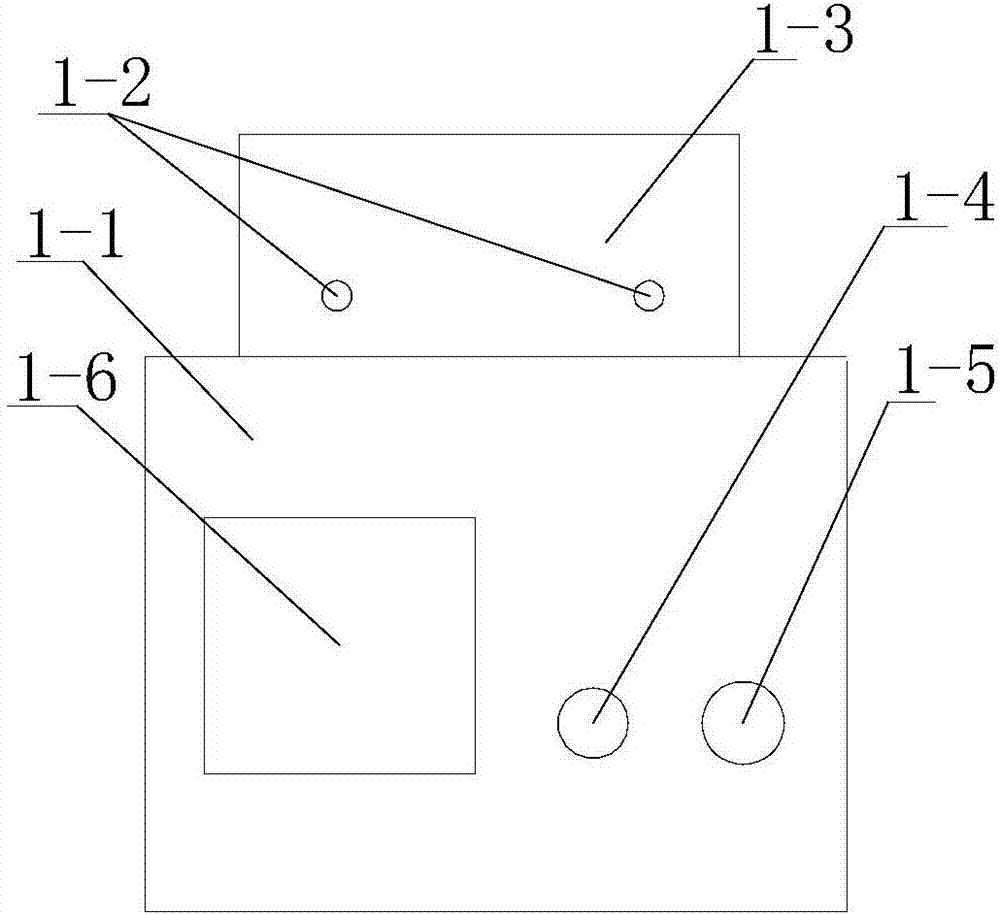
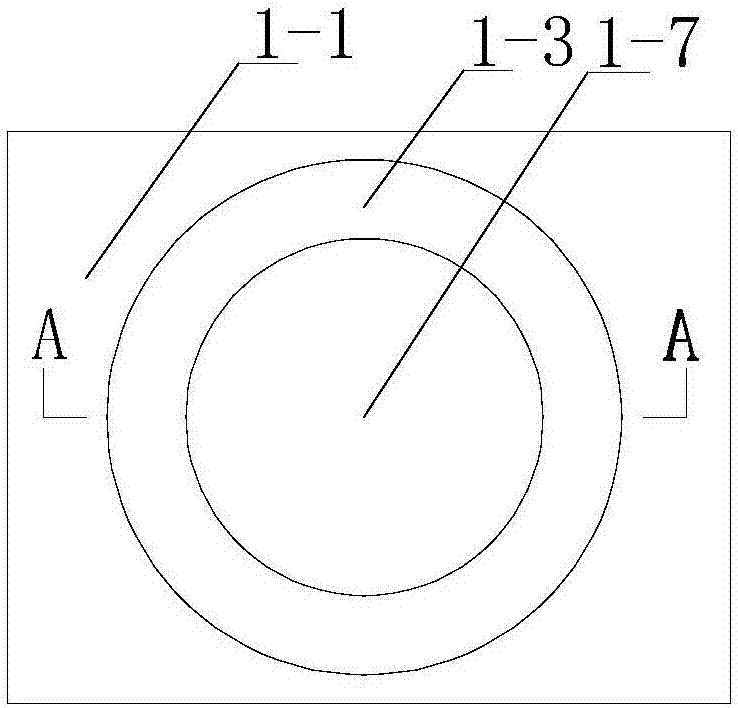
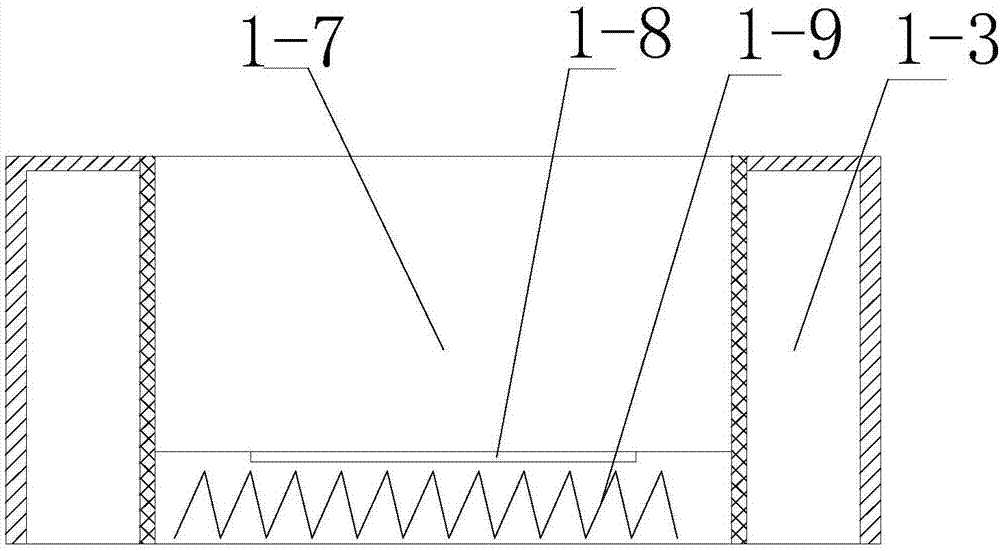
[0047] Among them such as Figure 1-6 As shown, the magnetic stirrer that can precisely control the temperature includes a base 1-1 and an oil bath 1-7 arranged on the base 1-1, and the outside of the oil bath 1-7 is provided with a vacuum chamber 1- 3, and the oil bath 1-7 is provided with a control system;
[0048] The vacuum chamber 1-3 is provided with two vacuum ports 1-2;
[0049] The control system includes a temperature control switch 1-4, a speed switch 1-5 and a display screen 1-6, the temperature of the oil bath is controlled by the temperature control switch 1-4, and the speed of magnetic stirring is controlled by the speed switch 1-5. The oil bath is provided with corresponding elect...
Embodiment 2
[0056] Utilize the combined reactor described in embodiment 1 to prepare the method for pyrazolylbenzaldehyde acetal p-phenylenediamine bis-Schiff base, concrete steps are as follows:
[0057] Weigh 0.57g (8.4mmol) of pyrazole, 2.32g (16.8mmol) of potassium carbonate, dissolve in 18.974g (259.6mmol) of N,N-dimethylformamide, add to the oven equipped with rotor, thermometer and In the 100mL three-necked flask of the dropping funnel of the slow-flow joint, react at 100°C for 30min under constant temperature stirring, and dropwise add p-fluorobenzaldehyde 1.04g (8.4mmol) to the mixed solution through the dropping funnel with the slow-flow joint, Continue the reaction for 10 hours, pass cold air into the vacuum chamber through two vacuum ports, and quickly cool the oil bath to room temperature to obtain the crude pyrazolylbenzaldehyde, recrystallize with absolute ethanol, and vacuum-dry at 50°C for 8 hours. That is, pyrazolylbenzaldehyde.
[0058] Weigh 0.11g (0.65mmol) of pyrazo...
Embodiment 3
[0060] Utilize the combined reactor described in embodiment 1 to prepare the method for pyrazolylbenzaldehyde acetal p-phenylenediamine bis-Schiff base, concrete steps are as follows:
[0061] Weigh 1.36g (20mmol) of pyrazole and 5.53g (40mmol) of potassium carbonate, dissolve them in 37.95g (519.2mmol) of N,N-dimethylformamide, and add them to a furnace equipped with a rotor, a thermometer and a device with slow flow. In the 100mL three-necked flask of the dropping funnel of the connector; after reacting for 30min under constant temperature stirring at 110°C, add 2.482g (20mmol) of p-fluorobenzaldehyde dropwise to the mixed solution through the dropping funnel with a slow flow connector, and continue the reaction After 12 hours, use a vacuum chamber to quickly cool to room temperature to obtain the crude product, recrystallize it with absolute ethanol, and dry it in vacuum at 50°C for 8 hours to obtain pyrazolylbenzaldehyde.
[0062] Weigh 0.172g (1mmol) of pyrazolylbenzaldeh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com