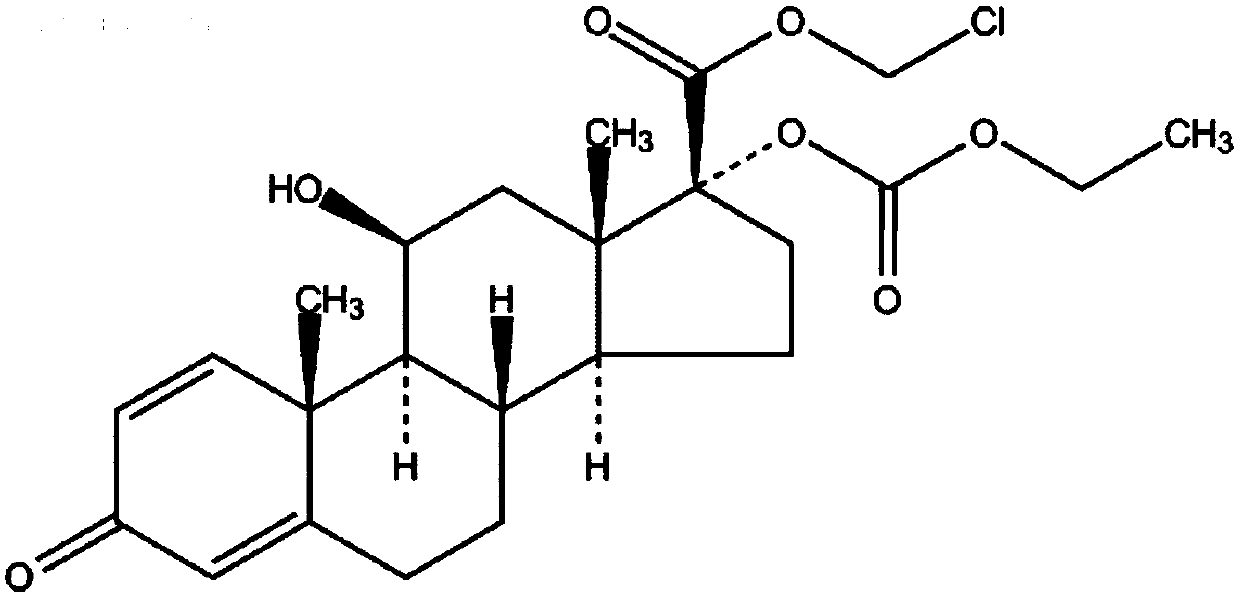
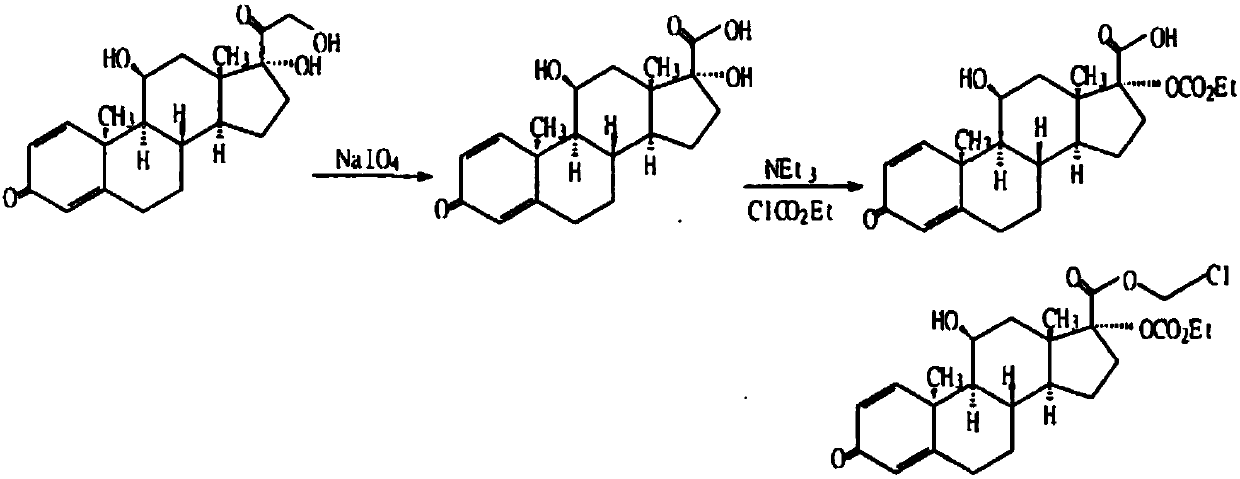
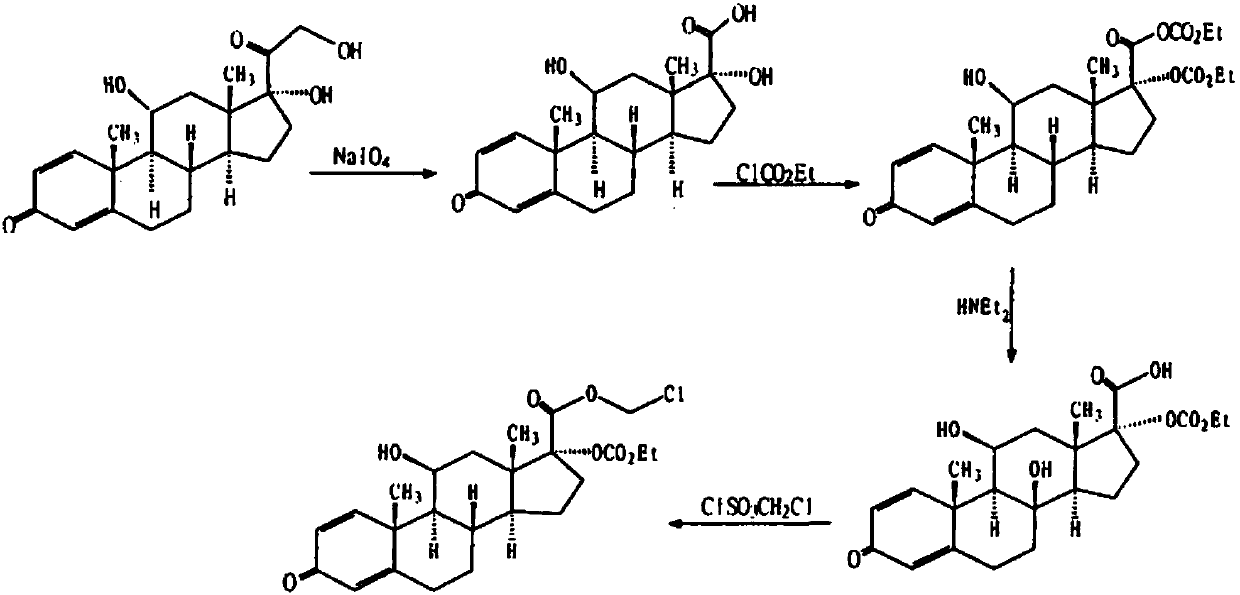
Process for preparing loteprednol and ophthalmic composition thereof
A technology of loteprednol and composition, which is applied in the direction of drug combination, steroids, drug delivery, etc., can solve the problems that cannot be ruled out, such as refrigerated storage, micronization of raw materials for preparation process and aggregation of eye drops particles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1: prepare loteprednol
[0085] Step 1. Oxidation
[0086]
[0087] In the mixed solution containing 120 milliliters of tetrahydrofuran and 30 milliliters of methanol, add 14.9 grams (40 mmol) of prednisolone, stir to dissolve, add dropwise warm sodium periodate solution (25.7 grams of sodium periodate are dissolved in 100 ml of water), after the dropwise addition, the reaction mixture was stirred at room temperature for 2 hours, concentrated under reduced pressure, distilled off tetrahydrofuran and methanol, cooled and suction filtered, washed with water, and dried to obtain 11α, 17β-dihydroxy-3-oxoandroster -1,4-diene-17β-carboxylic acid (Intermediate I) 13 grams, off-white powder, melting point 258-260°C, yield 90.8%.
[0088] Step 2. Condensation
[0089]
[0090] 10 grams of Intermediate I (28.8 mmol) were added to 100 milliliters of water containing 24.2 grams of sodium bicarbonate, stirred and dissolved, then 100 milliliters of dichloromethan...
Embodiment 2
[0099] Embodiment 2: prepare loteprednol
[0100] Step 1. Oxidation
[0101]
[0102] In the mixed solution containing 120 milliliters of tetrahydrofuran and 30 milliliters of methanol, add 14.9 grams (40 mmol) of prednisolone, stir to dissolve, add dropwise warm sodium periodate solution (25.7 grams of sodium periodate are dissolved in 100 ml of water), after the dropwise addition, the reaction mixture was stirred at room temperature for 3 hours, concentrated under reduced pressure, distilled off tetrahydrofuran and methanol, cooled and suction filtered, washed with water, and dried to obtain 11α, 17β-dihydroxy-3-oxoandroster 13.3 g of -1,4-diene-17β-carboxylic acid (Intermediate I), an off-white powder with a melting point of 258-261°C.
[0103] Step 2. Condensation
[0104]
[0105] 10 grams of Intermediate I (28.8 mmol) were added to 100 milliliters of water containing 24.2 grams of sodium bicarbonate, stirred and dissolved, then 100 milliliters of dichloromethan...
Embodiment 3
[0114] Embodiment 3: prepare loteprednol
[0115] Step 1. Oxidation
[0116]
[0117] In the mixed solution containing 120 milliliters of tetrahydrofuran and 30 milliliters of methanol, add 14.9 grams (40 mmol) of prednisolone, stir to dissolve, add dropwise warm sodium periodate solution (25.7 grams of sodium periodate are dissolved in 100 ml of water), after the dropwise addition, the reaction mixture was stirred at room temperature for 5 hours, concentrated under reduced pressure, distilled off tetrahydrofuran and methanol, cooled and suction filtered, washed with water, and dried to obtain 11α, 17β-dihydroxy-3-oxoandroster 13.3 g of -1,4-diene-17β-carboxylic acid (Intermediate I), an off-white powder with a melting point of 257-260°C.
[0118] Step 2. Condensation
[0119]
[0120] 10 grams of Intermediate I (28.8 mmol) were added to 100 milliliters of water containing 24.2 grams of sodium bicarbonate, stirred and dissolved, then 100 milliliters of dichloromethan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com