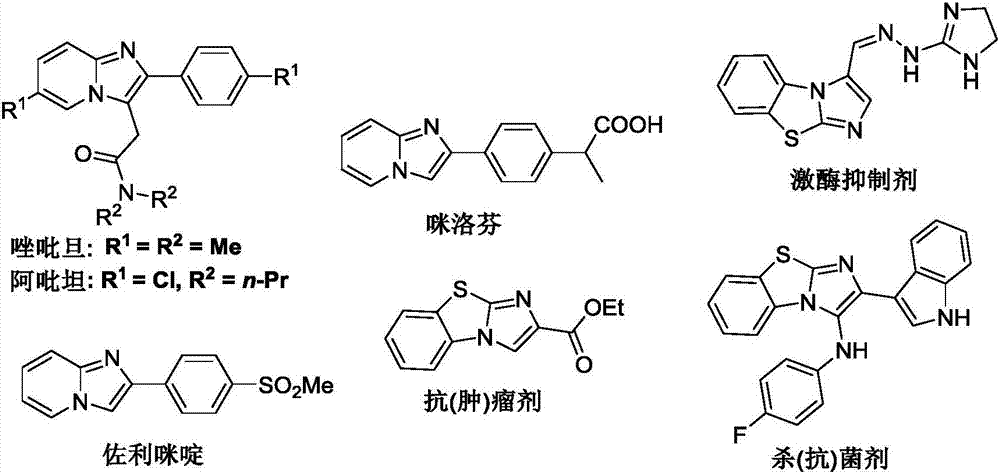
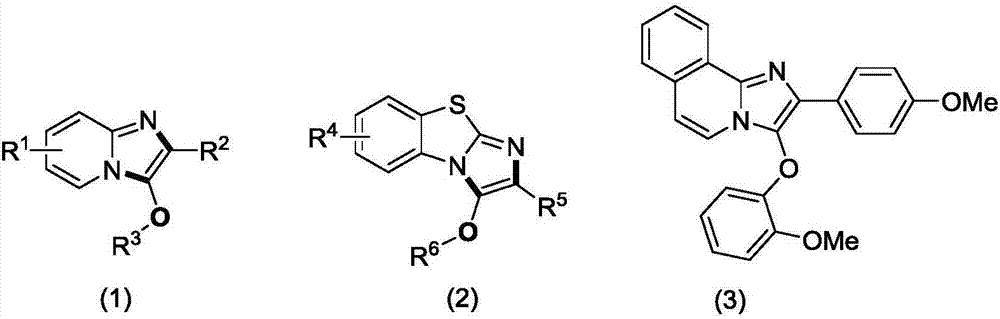
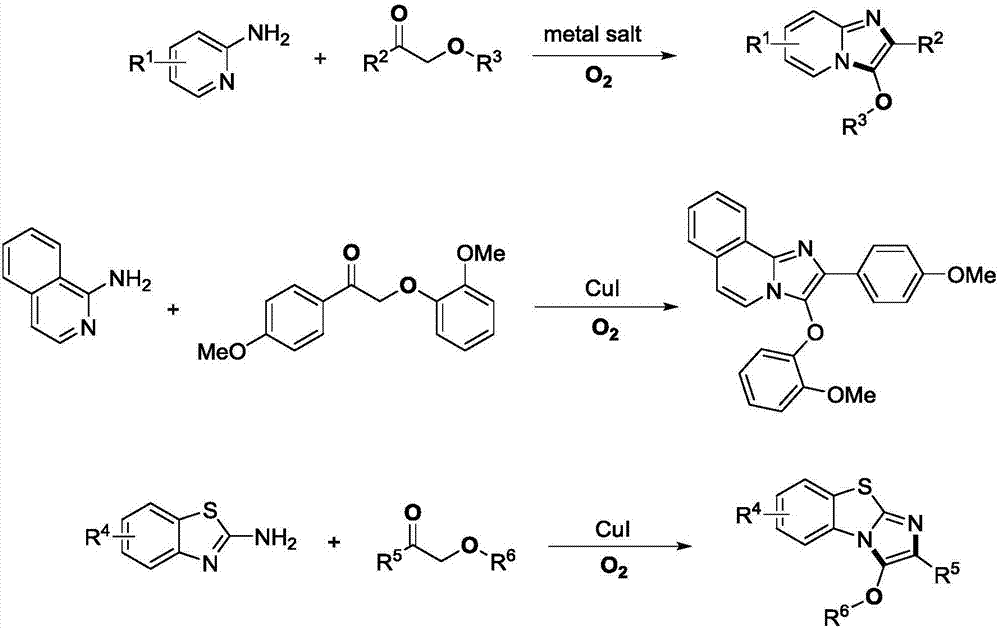
Method for preparing C-3 oxo substituted imidazole heterocyclic compounds
A technology of compounds and synthetic methods, applied in the fields of organic chemistry, drug combination, antibacterial drugs, etc., to achieve the effects of wide application prospect, high reaction yield and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 3-phenoxy-2-phenylimidazo[1,2-a]pyridine
[0032]
[0033] Take a clean reaction bottle, add a small magnet, dry it, add 2-aminopyridine (56.5mg, 0.6mmol), 2-phenoxyacetophenone (42.5mg, 0.2mmol), cuprous iodide (0.01mmol) , 1,2-dichloroethane (1.0 mL), heated at 100° C. under oxygen for 16 hours, and the reaction solution was separated by direct column chromatography to obtain the target product (44.0 mg, yield 77%). White solid, m.p.:145-146℃. 1 H NMR (400MHz, CDCl 3 ): δ=8.07(d, J=7.6Hz, 2H), 7.75(d, J=6.8Hz, 1H), 7.66(d, J=9.2Hz, 1H), 7.41(t, J=7.2Hz, 2H ),7.28-7.35(m,3H),7.20(t,J=7.6Hz,1H),7.12(t,J=7.2Hz,1H),6.97(d,J=8.0Hz,2H),6.77(t ,J=6.8Hz,1H). 13 C NMR (100MHz, CDCl 3 ):δ=156.1,139.9,132.5,131.1,130.2,128.7,127.8,126.5,124.4,123.7,121.6,117.9,115.0,112.3.FTIR(NaCl,cm -1 ):3053.3,2985.8,1647.2,1495.6,1421.5,1390.1,1362.2,1265.3,1201.7,895.0,738.7,706.0.HR-MS(ESI):m / zcalculated for C 19 h 14 N 2 O[M+H]+: 287.1184; found 287.1...
Embodiment 2
[0034] Example 2: Preparation of 3-phenoxy-2-phenylimidazo[1,2-a]pyridine
[0035]
[0036] Take a clean reaction bottle, add a small magnet, dry it, add 2-aminopyridine (56.5mg, 0.6mmol), 2-phenoxyacetophenone (42.5mg, 0.2mmol), cuprous iodide (0.01 mmol) , 1,2-dichloroethane (1.0 mL), heated at 80° C. under oxygen for 16 hours, and the reaction solution was separated by direct column chromatography to obtain the target product (40.0 mg, yield 70%).
Embodiment 3
[0037] Example 3: Preparation of 3-phenoxy-2-phenylimidazo[1,2-a]pyridine
[0038]
[0039] Take a clean reaction bottle, add a small magnet, dry it, add 2-aminopyridine (56.5mg, 0.6mmol), 2-phenoxyacetophenone (42.5mg, 0.2mmol), cuprous iodide (0.01mmol) , 1,2-dichloroethane (1.0 mL), heated at 120° C. under oxygen for 16 hours, and the reaction solution was separated by direct column chromatography to obtain the target product (37.8 mg, yield 66%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com