A kind of preparation method of 1,2-substituted benzimidazole derivatives
A technology of benzimidazoles and derivatives, applied in 1 field, can solve the problems of many side reactions and wastes, low atom utilization rate, unfriendly environment, etc., and achieves green post-treatment, small catalyst dosage, and reaction specificity. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of 2-phenyl-1-p-toluenesulfonylbenzimidazole, Ts = p-toluenesulfonyl
[0022]
[0023] N-phenylbenzamidine 0.2mmol, p-toluenesulfonyl azide 0.3mmol, dichloro(pentamethylcyclopentadienyl) iridium (IIl) dimer 0.008mmol, bistrifluoromethanesulfonimide Add 0.032mmol of silver salt and 0.2mmol of phenylacetic acid to a test tube containing a stirring bar and add 2mL of 1,2-dichloroethane solvent, place in an oil bath at 80°C, react for 12h, remove the heat source from the reaction, and cool to room temperature . The reaction solution was concentrated and purified by column chromatography to obtain 75.9 mg of the target product with a yield of 97%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl3) δ8.20 (d, J = 7.3Hz, 1H), 7.72 (d, J = 7.9Hz, 1H), 7.61 (d, J = 7.8Hz, 2H), 7.54 (dd, J =11.2, 4.5Hz, 1H), 7.48-7.36(m, 4H), 7.32(d, J=8.2Hz, 2H), 7.09(d, J=8.2Hz, 2H), 2.32(s, 3H). 13 C NMR (101MHz, CDCl3) δ 154.03, 145.64, 142.61, ...
Embodiment 2
[0025] Preparation of 4-fluoro-2-phenyl-1-p-toluenesulfonylbenzimidazole, Ts = p-toluenesulfonyl
[0026]
[0027] N-(2-fluorophenyl)benzamidine 0.2mmol, p-toluenesulfonyl azide 0.3mmol, dichloro(pentamethylcyclopentadienyl) iridium(III) dimer 0.008mmol, bistrifluoro Add 0.032mmol of methanesulfonylimide silver salt and 0.2mmol of phenylacetic acid to a test tube containing a stirring bar and add 2mL of 1,2-dichloroethane solvent, place it in an oil bath at 80°C, and react for 12 h. Remove from heat and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 47.2 mg of the target product, with a yield of 62%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.00(d, J=8.3Hz, 1H), 7.61(d, J=7.3Hz, 2H), 7.55(t, J=7.4Hz, 1H), 7.45(t, J=7.5Hz, 2H) , 7.36(td, J=8.3, 5.2 Hz, 1H), 7.31(d, J=8.2Hz, 2H), 7.09(t, J=7.6Hz, 3H), 2.32(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ154.22, 153.65 (d, J=25...
Embodiment 3
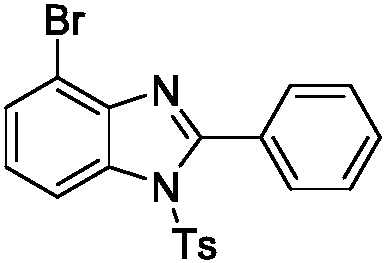
[0029] Preparation of 4-bromo-2-phenyl-1-p-toluenesulfonylbenzimidazole, Ts = p-toluenesulfonyl
[0030]
[0031] N-(2-bromophenyl)benzamidine 0.2mmol, p-toluenesulfonyl azide 0.3mmol, dichloro(pentamethylcyclopentadienyl) iridium(III) dimer 0.008mmol, bistrifluoro Add 0.032mmol of methanesulfonylimide silver salt and 0.2mmol of phenylacetic acid to a test tube containing a stirring bar and add 2mL of 1,2-dichloroethane solvent, place it in an oil bath at 80°C, and react for 12 h. Remove from heat and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 50.3 mg of the target product, with a yield of 60%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.18(d, J=8.3Hz, 1H), 7.60-7.56(m, 3H), 7.53(d, J=7.5Hz, 1H), 7.44(t, J=7.6Hz, 2H), 7.30( dt, J=8.0, 3.9 Hz, 3H), 7.10(d, J=8.3Hz, 2H), 2.33(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ154.42, 146.02, 141.22, 134.69, 134.30, 131.01, 130...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com