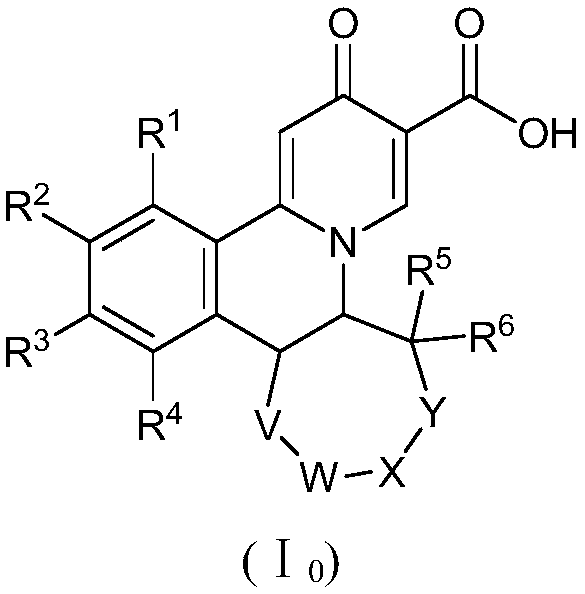
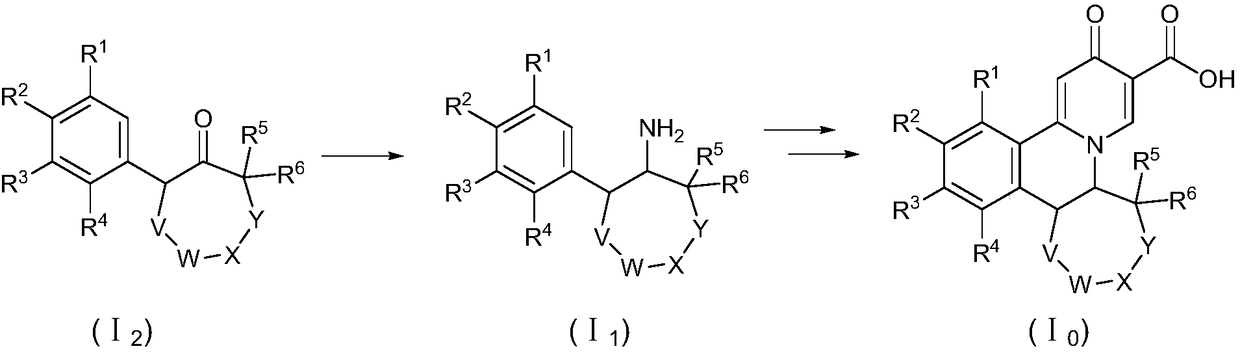
A kind of preparation method of quinazinone compound
A compound and quinazinone technology, applied in the field of preparation of quinazinones, can solve the problems of low compound stereoselectivity, difficult compound preparation, low stereoselectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
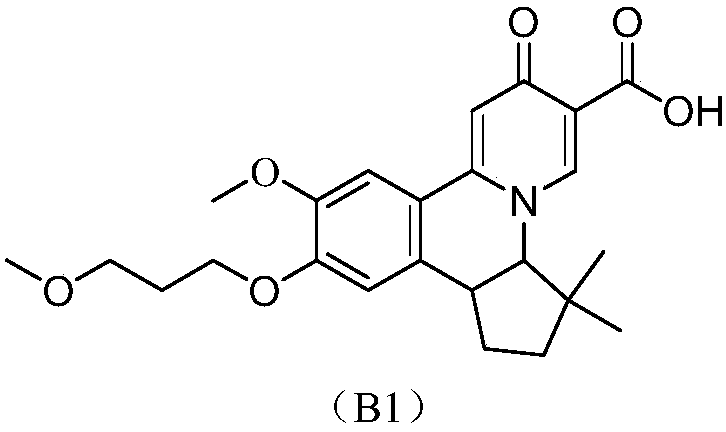
[0086] Example 1 cis-10-methoxy-11-(3-methoxypropoxy)-3,3-dimethyl-7-oxo-3,3a,7,12b-tetrahydro-2H- Preparation of Furo[3,2-c]pyrido[2,1-a]isoquinazine-6-carboxylic acid
[0087] The synthetic route is as follows:
[0088]
[0089] Step 1: Preparation of 6-(2-Hydroxy-1,1-dimethyl-ethyl)-10-methoxy-9-(3-methoxypropoxy)-2-oxo-6,7 -Ethyl dihydrobenzo[a]quinazine-3-carboxylate
[0090] 6-(2-Benzyloxy-1,1-dimethyl-ethyl)-10-methoxy-9-(3-methoxypropoxy)-2-oxo-6,7- Ethyl dihydrobenzo[a]quinazine-3-carboxylate (1.65g, 3mmol, preparation reference patent US20160122344A1), and Pd / C (10%) (0.5g) were slowly added to ethanol (200mL), under a hydrogen atmosphere Press down and stir mechanically to obtain crude 6-(2-hydroxy-1,1-dimethyl-ethyl)-10-methoxy-9-(3-methoxypropoxy)-2- Ethyl oxo-6,7-dihydrobenzo[a]quinazine-3-carboxylate (1.10 g, yield about 79.7%) was directly used in the next step without purification.
[0091] 1 H NMR (400MHz, CDCl 3 )δ:8.43(s,1H),7.12(s,1H),6.89(s,1H),6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com