Skin allergen patch test diagnosis matrix composition and preparation method thereof
A matrix composition, skin allergy technology, applied in preparations for in vivo experiments, compound screening/testing, pharmaceutical formulations, etc., can solve the problems of low sensitivity, troublesome operation, long detection time, etc., to enhance sensitivity, reduce Barrier effect, effect of shortening detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] A preparation method of a skin allergen patch test diagnostic matrix composition, comprising the steps of:
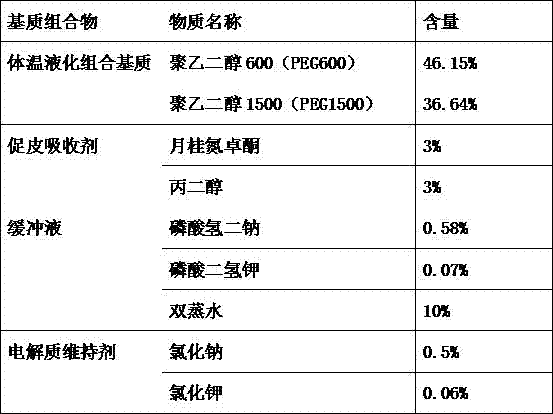
[0075] 1) Preparation of body temperature liquefaction combination matrix: First, put the body temperature liquefaction combination matrix accounting for 80-90% of the composition mass percentage in a 60-80°C constant temperature water bath to dissolve, mix well, and then set the temperature of the constant temperature water bath is 45°C. The body temperature liquefaction combination matrix is composed of polyethylene glycol (PEG) and / or propylene glycol, wherein polyethylene glycol (PEG) includes polyethylene glycol 300 (PEG300), polyethylene glycol 400 (PEG400), polyethylene glycol Glycol 600 (PEG600), polyethylene glycol 1000 (PEG1000), polyethylene glycol 1450 (PEG1450), polyethylene glycol 1500 (PEG1500), polyethylene glycol 2000 (PEG2000), polyethylene glycol 3305 (PEG3305) ), polyethylene glycol 4000 (PEG4000), polyethylene glycol 6000 (PEG6000).
[00...
Embodiment 1
[0086] A skin allergen patch test diagnostic matrix composition, comprising the following components according to the mass percentage of the composition:
[0087]
[0088] A preparation method of a skin allergen patch test diagnostic matrix composition corresponding to the component, comprising the steps of:
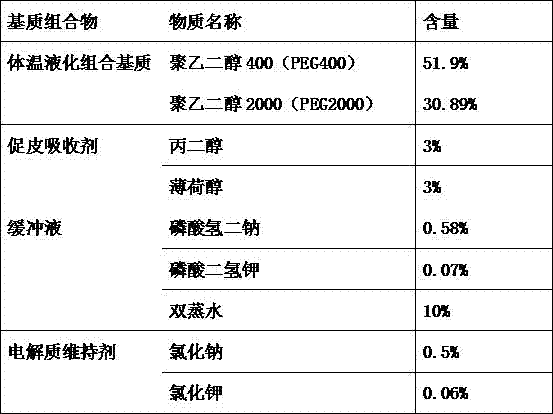
[0089]1) Prepare body temperature liquefaction combination matrix: first dissolve polyethylene glycol 1500 (PEG1500) in a constant temperature water bath at 60°C, then add polyethylene glycol 600 (PEG600) and mix well, then set the temperature of the constant temperature water bath to 45 ℃;
[0090] 2) Add skin absorption promoting agent: add laurocapram and propylene glycol to the body temperature liquefaction combination matrix in step 1 and mix well for later use;
[0091] 3) Preparation of buffer solution: Dissolve disodium hydrogen phosphate and potassium dihydrogen phosphate in double distilled water, and measure its pH value to 7.4;
[0092] 4) Add electrolyt...
Embodiment 2
[0099] A skin allergen patch test diagnostic matrix composition, comprising the following components according to the mass percentage of the composition:
[0100]
[0101] A preparation method of a skin allergen patch test diagnostic matrix composition corresponding to the component, comprising the steps of:
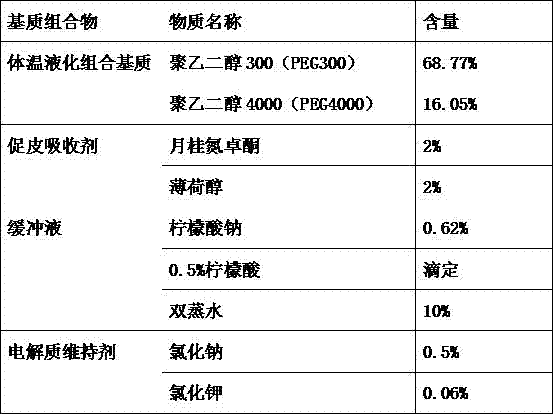
[0102] 1) Preparation of body temperature liquefaction combination matrix: first melt polyethylene glycol 2000 (PEG2000) in a constant temperature water bath at 60-70°C, then add polyethylene glycol 400 (PEG400) and mix thoroughly, then set the temperature of the constant temperature water bath 45°C;
[0103] 2) Add skin absorption agent: add propylene glycol and menthol to the body temperature liquefaction combination matrix in step 1 and mix well for later use;
[0104] 3) Preparation of buffer solution: Dissolve disodium hydrogen phosphate and potassium dihydrogen phosphate in double distilled water, and measure its pH value to 7.4;
[0105] 4) Add electrolyte ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com