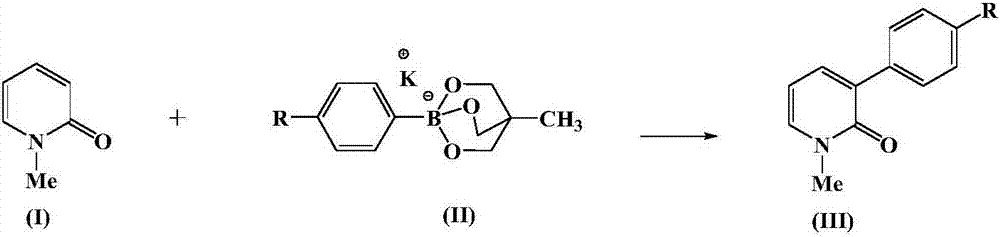
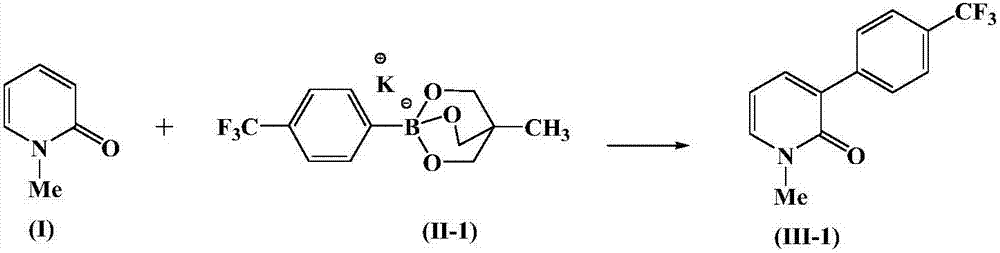
Aryl pyridone compound preparation method
A technology of aryl pyridone and compound, which is applied in the field of pharmaceutical and organic chemical synthesis, can solve the problems affecting large-scale production and application, low yield, complicated operation, etc., and achieve the effect of good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] At 0°C, add 1 mmol N-methyl-2-pyridone, 1.2 mmol formula (II-1) compound, 0.1 mmol bis(1,5-cyclooctadiene) rhodium tetrafluoroborate to the reactor , 0.1 mmol of Ligand L, 8 ml of N,N-dimethylformamide and 2 ml of toluene, stirred at 60°C for 6 hours. After the reaction was completed, the reaction mixture was poured into water and extracted with ethyl acetate, the organic phase was combined to remove the solvent, and the resulting residue was separated by silica gel column chromatography to obtain the compound of formula (III-1) with a yield of 89.5%. 1 HNMR (CDCl 3 ,400MHz): δ3.61(s,3H),6.27(dd,J=6.9,6.9Hz,1H),7.35(dd,J=2.0,6.9Hz,1H),7.53(dd,J=2.0,6.9 Hz, 1H), 7.62 (d, J=8.2Hz, 2H), 7.81 (d, J=8.2Hz, 2H).
Embodiment 2
[0028]
[0029] At 0°C, add 1 mmol N-methyl-2-pyridone, 1.5 mmol formula (II-2) compound, 0.1 mmol bis(1,5-cyclooctadiene) rhodium tetrafluoroborate to the reactor , 0.1 mmol of Ligand L, 6 ml of N,N-dimethylformamide and 4 ml of toluene, stirred at 60°C for 6 hours. After the reaction was completed, the reaction mixture was poured into water and extracted with ethyl acetate, the organic phase was combined to remove the solvent, and the resulting residue was separated by silica gel column chromatography to obtain the compound of formula (III-2) with a yield of 85.2%. 1 HNMR (CDCl 3 ,400MHz): δ2.37(s,3H),3.60(s,3H),6.22(dd,J=6.9,6.9Hz,1H),7.20(d,J=7.9Hz,2H),7.28(dd, J=2.0, 6.9Hz, 1H), 7.46(dd, J=2.0, 6.9Hz, 1H), 7.59(d, J=7.9Hz, 2H).
Embodiment 3
[0031] At a temperature of 0°C, 1 mmol of N-methyl-2-pyridone, 1.2 mmol of the compound of formula (II-1), 0.1 mmol of bis(1,5-cyclooctadiene) rhodium chloride, 0.1 mmol of Ligand L, 8 ml of N,N-dimethylformamide and 2 ml of toluene were stirred at 60°C for 6 hours. After the reaction was completed, the reaction mixture was poured into water and extracted with ethyl acetate, the organic phase was combined to remove the solvent, and the resulting residue was separated by silica gel column chromatography to obtain the compound of formula (III-1) with a yield of 86.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com