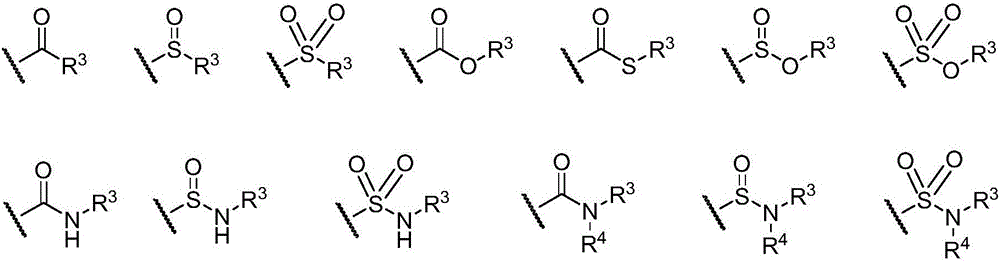
Bruton's tyrosine kinase inhibitor
An amino and solvate technology, applied in the field of medicine, can solve the problems of no significant prolongation of the overall survival of patients, high toxicity, and frequent occurrence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] Synthetic route of compound 16-1
[0167]
[0168] N-((3-chloropyrazin-2-yl)methyl)-(7-benzyloxycarbonyl-7-aza-bicyclo[2.2.1]heptane-1)-carboxamide 10
[0169]
[0170] To a solution of compound 9 (3g, 10.9mmol) and (3-chloropyrazin-2-yl)methanamine hydrochloride (1.96g, 10.9mmol) in dichloromethane (50ml) was added DIEA (5.62g, 43.6mmol) ). It was stirred at this temperature for 15 minutes, HATU (4.35 g, 11.44 mmol) was added and stirred at 0 °C for a further 1 hour. It was then stirred overnight at room temperature. Pour into water (100ml) and separate the organic phase. The aqueous phase was extracted with dichloromethane (2 x 30ml). The organic phase was separated, dried over anhydrous Na2SO4, filtered and evaporated. The residue was purified by column chromatography, eluting with ethyl acetate / petroleum ether=1:5 to obtain 3.12 g of compound 10, yield: 71%.
[0171] 1H NMR (400MHz, CDCl3): δ1.50-1.60(m,2H); 1.85-2.10(m,4H); 2.15-2.25(m,2H); 2.40-2.60(m,...
Embodiment 2
[0197] 4-(8-Amino-3-(7-but-2-ynoyl-7-aza-bicyclo[2.2.1]heptane-1-yl)imidazo[1,5-a]pyrazine-1 -yl)-N-(4-fluoropyridin-2-yl)benzamide 16-2
[0198]
[0199] Synthesis of Compound 16-2 Using intermediate 13 and B-2 as raw materials, it was synthesized using Synthesis Method 1 of 16-1. After purification, 7 mg of compound 16-2 was obtained. LC-MS m / z=510.2[M+1] + .
Embodiment 3
[0201] 4-(8-Amino-3-(7-but-2-ynoyl-7-aza-bicyclo[2.2.1]heptane-1-yl)imidazo[1,5-a]pyrazine-1 -yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide 16-3
[0202]
[0203]Synthesis of Compound 16-3 Using intermediate 13 and B-3 as raw materials, it was synthesized using Synthesis Method 1 of 16-1. After purification, 11 mg of compound 16-3 was obtained. LC-MS m / z=560.2[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com