Self-supporting phosphating nickel nanomaterial for hydrogen production with electrocatalytic decomposition of water and preparation method of self-supporting phosphating nickel nanomaterial
A technology of nanosheets and nickel phosphide, applied in chemical instruments and methods, electrolytic processes, electrolytic components, etc., can solve the problems of few active sites and poor stability, and achieve simple operation, good stability, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
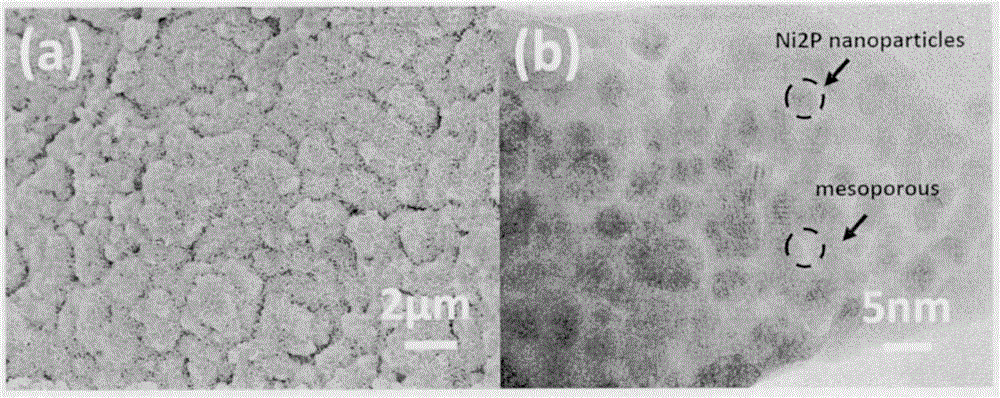
[0025] The commercial nickel mesh (1×3 cm in size) was washed successively with ethanol, acetone and deionized water, and then dried at 100° C. for 12 hours. The obtained clean nickel mesh was placed in a 50 mL reactor containing 30 mL of hydrogen peroxide (10 wt %) solution. The sealed reactor was placed at 120°C for 2 hours, and after the reaction was finished, it was naturally cooled down to room temperature. After the product was washed, it was further dried at 100°C and marked as Ni / NF. The obtained Ni / NF was confirmed to be nickel hydroxide nanosheets by XRD and SEM characterization, and it grew directly on the surface of nickel mesh. The obtained nickel mesh after oxidation etching was placed in the middle of the tube furnace, 300 mg of sodium hypophosphite was placed in the upper part of the tube furnace, under the protection of nitrogen, the nitrogen flow rate was 50 mL / min, the temperature was programmed to 350 ° C for 2 hours, and the temperature was raised The rat...
Embodiment 2
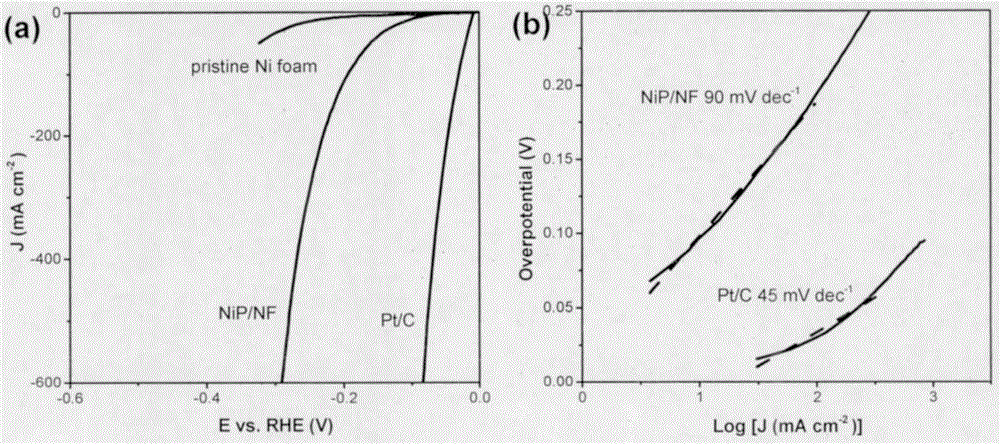
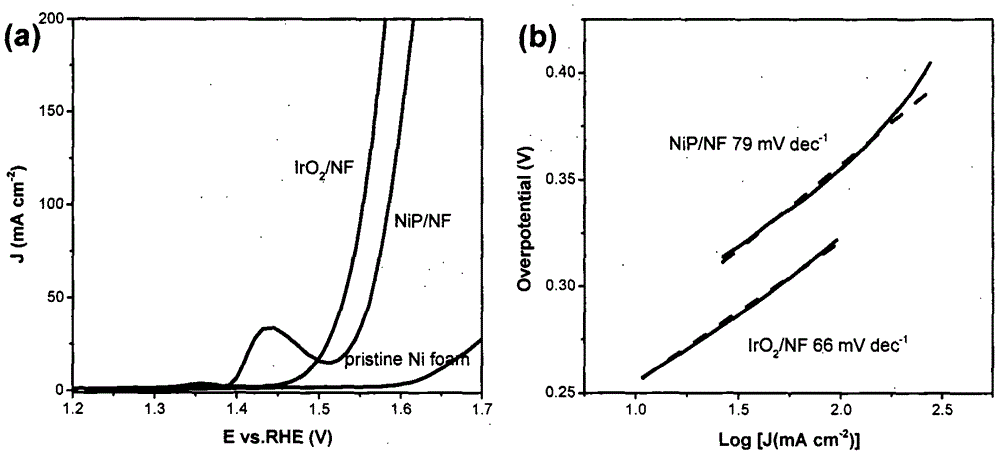
[0027] The sample NiP / NF in Example 1 was directly used as the working electrode. The electrochemical test was carried out on a computer-controlled electrochemical workstation, using a three-electrode test system, Ag / AgCl as the counter electrode, Pt wire as the working electrode, and 1.0M KOH aqueous solution as the alkaline electrolyte. The test range of the polarization curve is 0 to -0.6V for HER (vs reversible hydrogen electrode), and 1.2 to 1.7V for OER (vs reversible hydrogen electrode). image 3 with Figure 4 The linear polarization curves and Tafel curves of the synthesized catalysts are shown for the HER and OER processes, respectively.
Embodiment 3
[0029] The sample NiP / NF in Example 2 was directly used as the cathode and the anode respectively, and under the condition of the two-electrode test system, 1.0M KOH aqueous solution was used as the alkaline electrolyte. Electrochemical tests were performed on a computer-controlled electrochemical workstation. The scanning range of the electrodes is 1.1 to 2.0V. Figure 5 The linear sweep polarization curves of the synthesized catalysts are shown. It can be seen from the figure that when the voltage is 1.62V, the catalytic system can reach 10mA / cm 2 The current density of the catalyst shows the excellent electrocatalytic activity of hydrogen evolution and oxygen evolution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com