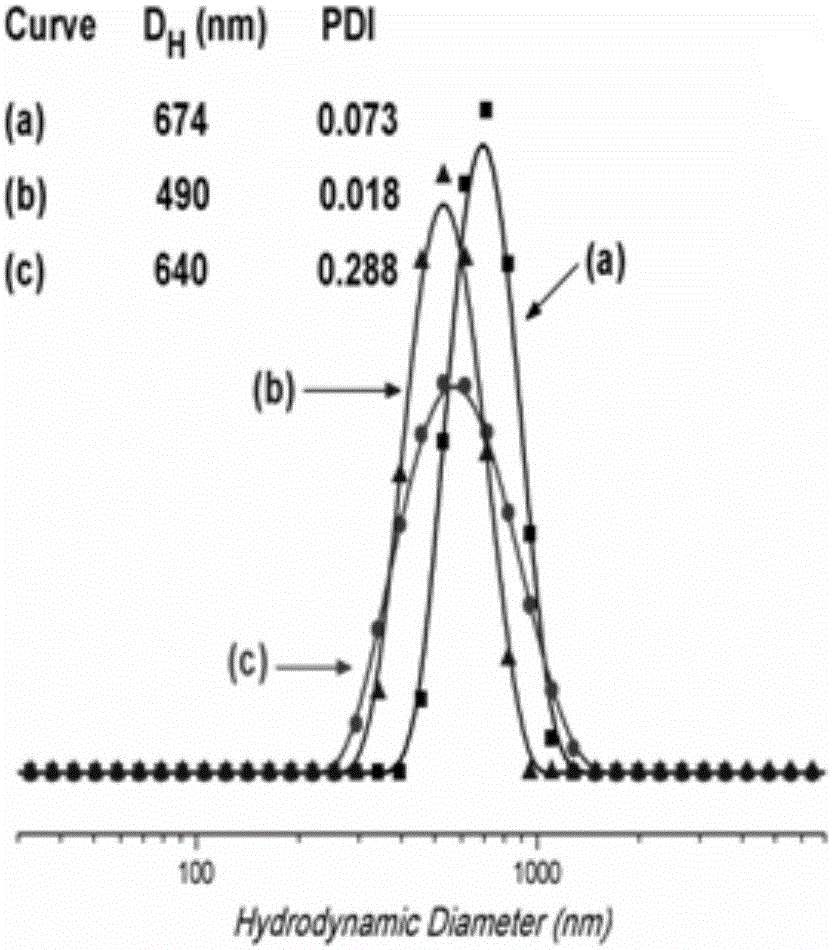
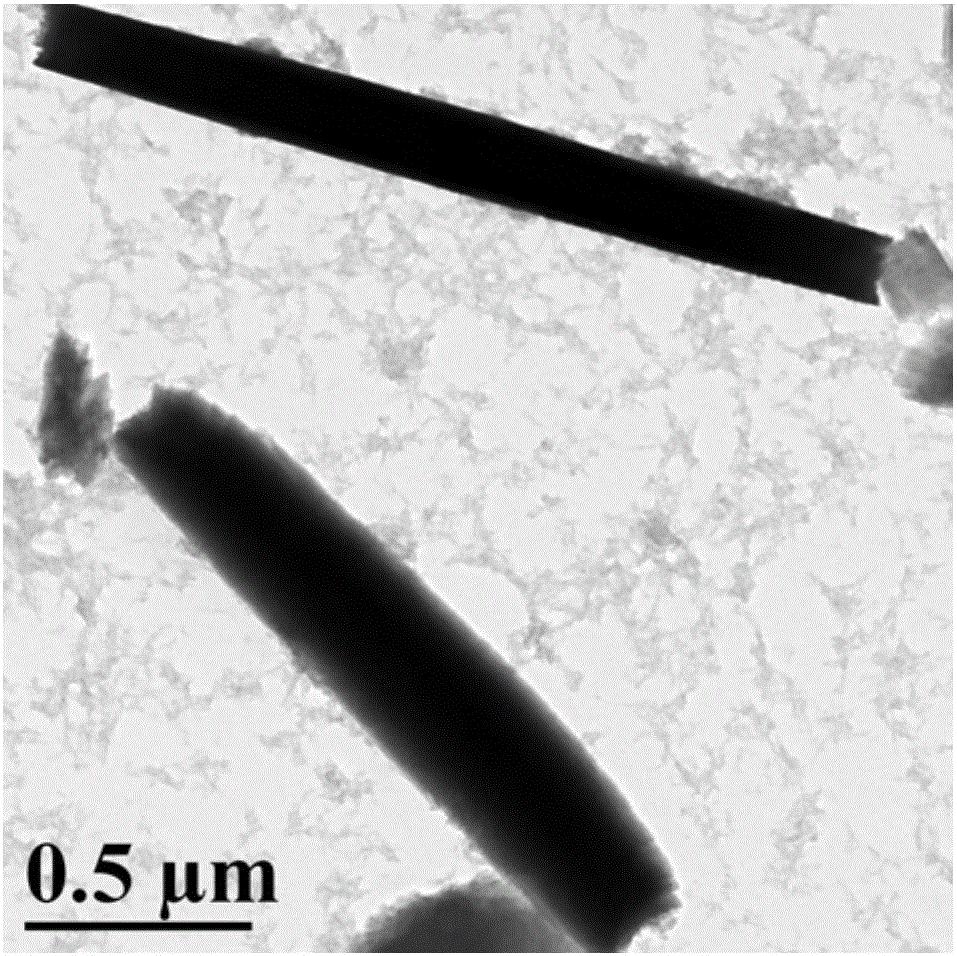
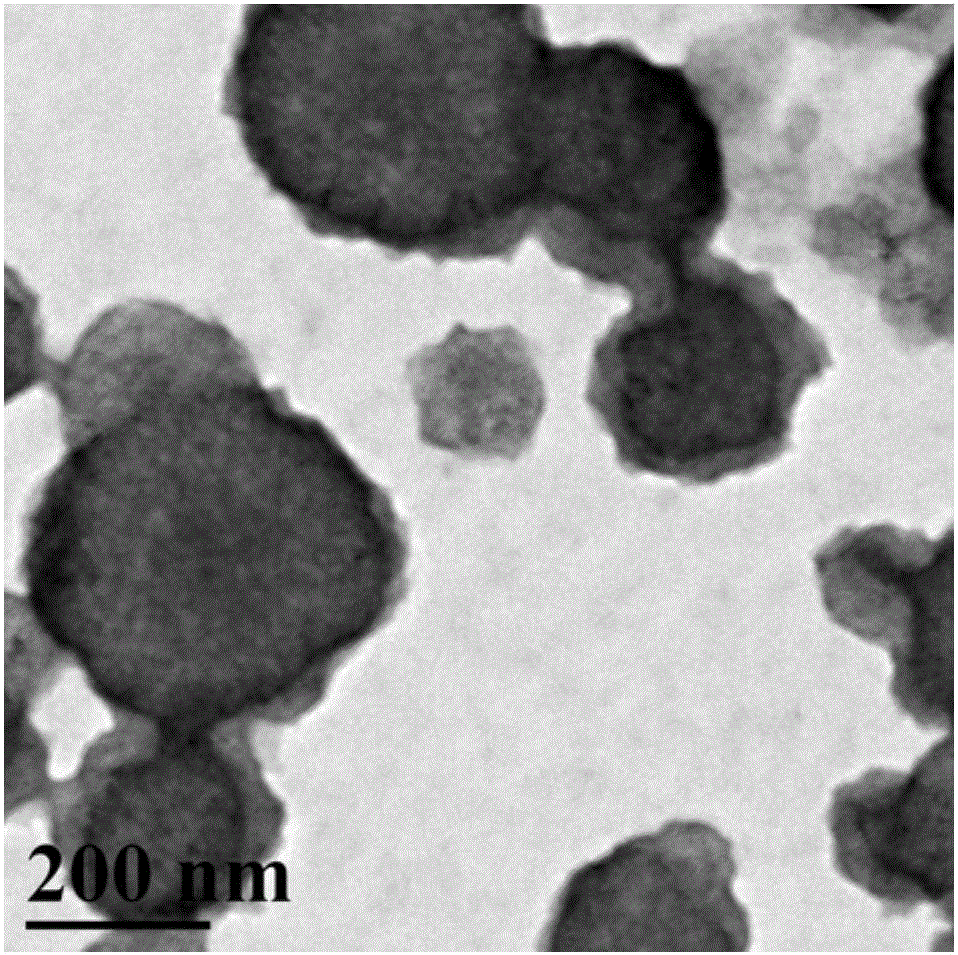
Synthesis of amphipathic block antibacterial peptide as well as preparation method and application of assembly of amphipathic block antibacterial peptide
An amphiphilic block and antimicrobial peptide technology, which is applied in the field of synthesis and assembly preparation, can solve the problems of unsatisfactory antibacterial effect, poor biocompatibility, limitations, etc., and achieve wide application prospects and value. control, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075]
[0076] A preparation method of the above-mentioned amphiphilic block antimicrobial peptide, which comprises the steps of:
[0077] (1), react the hydrophilic amino acid containing the amino protecting group and triphosgene in an organic solvent to obtain a hydrophilic amino acid-N-carboxyl-α-amino acid anhydride monomer, and combine the hydrophobic amino acid and triphosgene in an organic solvent Reaction in solvent to obtain hydrophobic amino acid-N-carboxyl-α-amino acid anhydride monomer;
[0078] (2), mix 0.200-1.000mmol initiator and 0.200-100.000mmol hydrophilic amino acid-N-carboxyl-α-amino acid anhydride monomer in 20-50mL organic solvent, and react under vacuum to obtain:
[0079] Wherein A represents an initiator, and Z' represents an amino protecting group;
[0080] (3), continue to add hydrophobic amino acid-N-carboxyl-α-amino acid anhydride monomer and react under vacuum to obtain:
[0081]
[0082] (4), repeat steps (2) and (3) n-1 times, obtain ...
Embodiment 1
[0129] The first step: preparation method of amphiphilic diblock antimicrobial peptide
[0130] The preparation method of the amphiphilic diblock antimicrobial peptide of the present embodiment, it comprises the steps:
[0131] (1) 30.000g (107.14mmol) benzyloxycarbonyl‐lysine and 13.790g (46.43mmol) triphosgene were subjected to a nucleophilic reaction in 40mLDMF at a temperature of 40°C for 3h to obtain Z'‐Lys‐NCA Monomer (Z' represents the benzyloxycarbonyl protecting group), 30.000g (181.18mmol) of phenylalanine and 23.400g (78.78mmol) of triphosgene in 40mL of DMF through nucleophilic reaction, the temperature is 40 ° C, the time is 3h, Obtain Phe-NCA monomer;
[0132] (2), 0.022mL (0.217mmol) isobutylamine (as an initiator) and 2.000g (6.540mmol) Z'‐Lys‐NCA monomer (Z' represents the benzyloxycarbonyl protecting group) was dissolved in 20mL (260mmol) Carry out ring-opening polymerization in DMF, react under vacuum for 12 hours at a temperature of 50°C, and obtain a hyd...
Embodiment 2
[0160] The first step: preparation method of amphiphilic diblock antimicrobial peptide
[0161] The preparation method of the amphiphilic diblock antimicrobial peptide of the present embodiment, it comprises the steps:
[0162] (1) 30.000g (107.14mmol) benzyloxycarbonyl‐lysine and 13.790g (46.43mmol) triphosgene were subjected to a nucleophilic reaction in 40mL DMF at a temperature of 40°C for 3 hours to obtain Z'‐Lys‐ NCA monomer (Z' represents the benzyloxycarbonyl protecting group), 30.000g (181.18mmol) of phenylalanine and 23.400g (78.78mmol) of triphosgene in 40mL of DMF by nucleophilic reaction, the temperature is 40 ℃, the time is 3h , to obtain the Phe-NCA monomer;
[0163] (2), 0.022mL (0.217mmol) isobutylamine (as an initiator) and 2.000g (6.540mmol) Z'‐Lys‐NCA monomer (Z' represents the benzyloxycarbonyl protecting group) were dissolved in 20mL DMF for development Ring polymerization, reacting under vacuum for 12 hours at a temperature of 50°C to obtain a hydrophi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com