Preparation technology of lithium carbonate
A preparation process, lithium carbonate technology, applied in the field of lithium extraction from brine, can solve the problems of pollution, complex process, high cost, etc., and achieve high economic benefits, high quality, and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present embodiment provides a kind of preparation technology of lithium carbonate, comprises the steps:
[0033] Step S1 impurity removal and concentration process:
[0034] Step a1: First, the salt lake brine is sent into an adsorption-desorption device equipped with a lithium adsorbent, so that the salt lake brine undergoes successive adsorption, leaching and desorption operations to obtain a lithium desorption solution. The lithium ion concentration in the lithium desorption solution is 0.4-0.6g / L, the magnesium ion concentration is 0.5-1.5g / L, the sodium ion concentration is 0.3-0.5g / L, and the chloride ion concentration is 2.5-4.0g / L .
[0035] The above-mentioned adsorption, rinsing, and desorption operations on salt lake brine are well known to those skilled in the art, so they will not be described in detail here. The lithium adsorbents used in this example include but are not limited to manganese-based adsorbents, aluminum-based adsorbents Adsorbents, tit...
Embodiment 1
[0067] Step S1 impurity removal and concentration process:
[0068] Step a1: sending the salt lake brine into an adsorption-desorption device equipped with a lithium adsorbent, so that the salt lake brine undergoes successive adsorption, leaching and desorption operations to obtain a lithium desorption solution. The lithium ion concentration in the lithium desorption solution was 0.4 g / L, the magnesium ion concentration was 0.5 g / L, the sodium ion concentration was 0.3 g / L, and the chloride ion concentration was 2.5 g / L.
[0069] Step b1: passing the above lithium desorption solution through the calcium and magnesium adsorption resin, thereby removing magnesium ions in the lithium desorption solution, so that the magnesium ion concentration of the lithium desorption solution is 0.018 mg / L, and obtaining the impurity removal solution.
[0070] Step c1: using the reverse osmosis concentration method to concentrate the impurity-removing liquid to obtain a section of lithium conce...
Embodiment 2
[0083] Each step in the present embodiment is identical with embodiment one, just in the present embodiment, what sodium carbonate material adopts is not the by-product sodium carbonate decahydrate crystal in the urea method to produce biurea process, but soda ash solution, therefore omits For the treatment steps a2-b2 of the by-product sodium carbonate decahydrate, the soda ash solution and the lithium-containing concentrated solution are directly mixed and reacted.
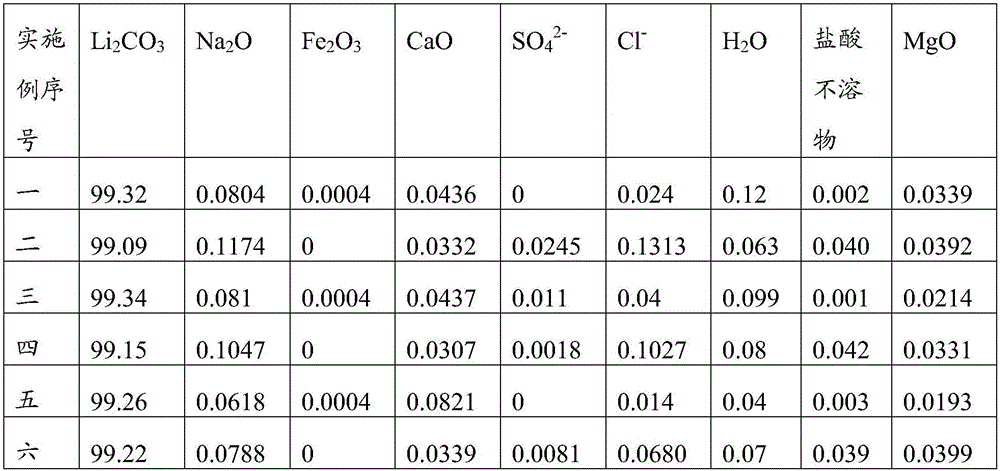
[0084] In the produced lithium carbonate product of the present embodiment, the massfraction of lithium carbonate is 99.09%, and impurity content is as follows: Na 2 The mass fraction of O is 0.1174%, Fe 2 o 3 The mass fraction of CaO is 0, the mass fraction of CaO is 0.0332, and the mass fraction of SO 4 2- The mass fraction of Cl is 0.0245, Cl - The mass fraction of 0.1313%, H 2 The mass fraction of O is 0.063%, the mass fraction of hydrochloric acid insoluble matter is 0.040%, and the mass fraction of Mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com