Aza-acridine compound and preparation method and application thereof
A compound and solvate technology, applied in the field of azaacridine parent nucleus synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0107] Example 1A: (Compound I) Preparation
[0108] Step 1a, 1b prepares 2-aminoquinoline-3-carbonitrile (2)
[0109] O-nitrobenzaldehyde (3.02g, 20mmol) was dissolved in EtOH (20ml), then iron powder (3.0g) and ammonium chloride (1.07g, 20mmol) aqueous solution (5ml) were added thereto, and the reaction was refluxed. After completion of the reaction, filter while hot, collect the filtrate, directly add malononitrile (1.32g, 20mmol) and Et 3 N (2ml), heated to reflux for reaction. Then the reaction solution was cooled to room temperature, and the solid was collected by filtration as compound 2. Yield 79.6%.
[0110] The confirmed data of the compound structure are:
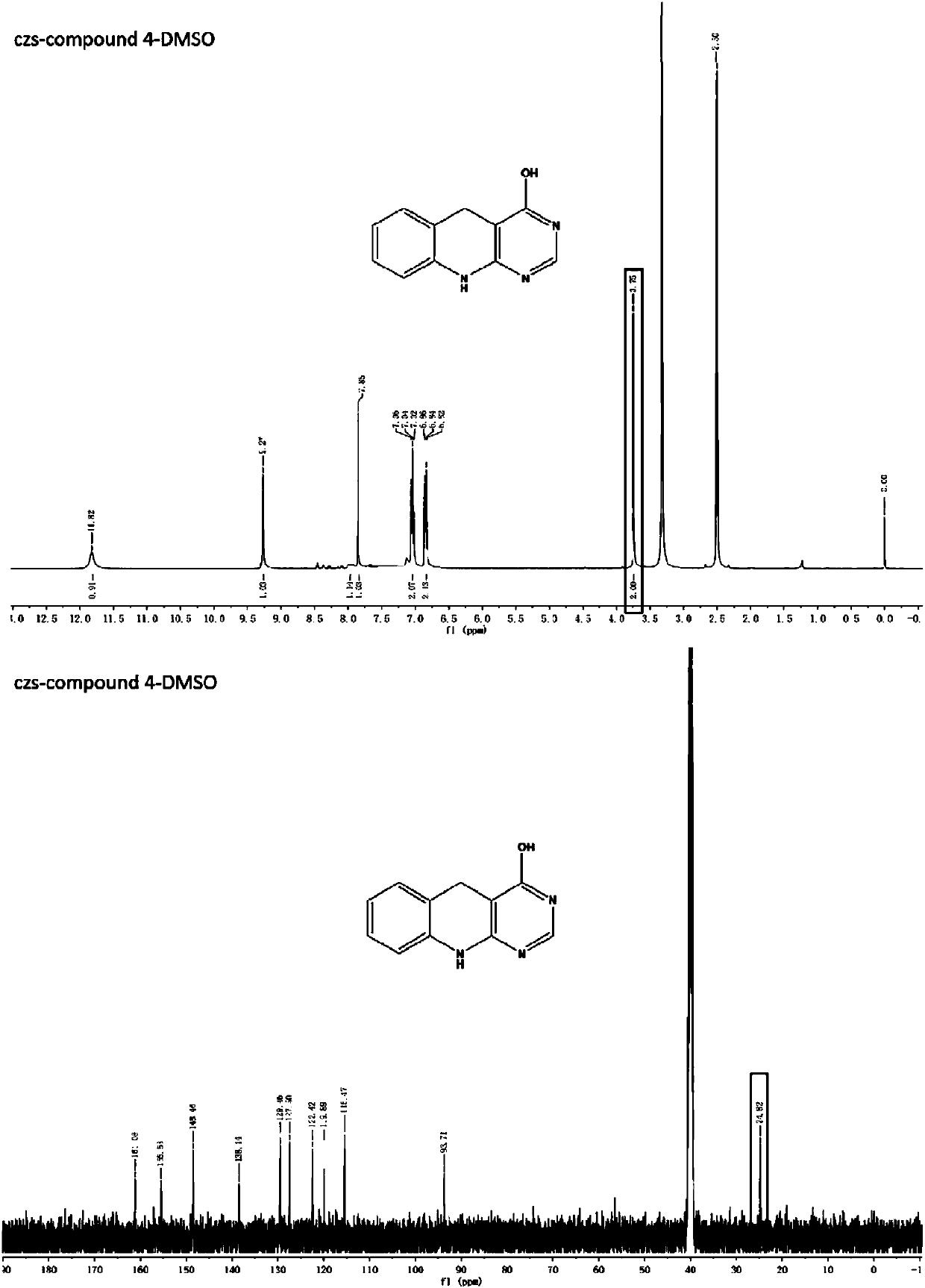
[0111] 1 H NMR (400MHz, DMSO) δ8.69(s, 1H), 7.65(d, J=8Hz, 1H), 7.66(dd, J 1 =J 2 =8Hz,1H),7.51(d,J=8Hz,1H),7.28(dd,J 1 =J 2 =8Hz,1H),7.21(s,3H).
[0112] Step 1c, preparation of 2-aminoquinoline-3-carboxamide (3)
[0113] Compound 2 (1.69 g, 10 mmol) was dissolved in concentrated H 2 SO 4 (10ml), ...
Embodiment 1
[0120] Example 1: (Compound 14a) Preparation
[0121] Step 2a, 2b prepare 2-aminoquinoline-3-formonitrile (2)
[0122] O-nitrobenzaldehyde (3.02g, 20mmol) was dissolved in EtOH (20ml), then iron powder (3.0g) and ammonium chloride (1.07g, 20mmol) aqueous solution (5ml) were added thereto, and the reaction was refluxed. After completion of the reaction, filter while hot, collect the filtrate, directly add malononitrile (1.32g, 20mmol) and Et 3 N (2ml), heated to reflux for reaction. Then the reaction solution was cooled to room temperature, and the solid was collected by filtration as compound 2. Yield 79.6%.
[0123] Step 2c, preparation of 2-aminoquinoline-3-carboxamide (3)
[0124] Compound 2 (1.69 g, 10 mmol) was dissolved in concentrated H 2 SO 4 (10ml), stirred for 24h, cooled the reaction solution and added it dropwise to ice water, adjusted the pH to about 8 with 20% NaOH solution, collected the precipitate by filtration, and dried to obtain compound 3. Yield 9...
Embodiment 2
[0141] Example 2: (Compound 14b) Preparation
[0142] Compound 14b was prepared according to the steps of Example 1, except that:
[0143] 1. Preparation of 1-(3-fluorophenyl)-3-(4-nitrophenyl)urea (7b)
[0144]The aniline in step 1g in Example 1 was replaced with 3-fluoroaniline for reaction. Yield 83.9%.
[0145] 2. Preparation of 1-(4-aminophenyl)-3-(3-fluorophenyl)urea (8b)
[0146] Replace 7a in step 1h in Example 1 with 7b for reaction. Yield 87.1%.
[0147] 3. Preparation of 1-(4-((5,10-dihydropyrimidinone[4,5-b]quinoline-4-)amino)phenyl)-3-(3-fluorophenyl)urea (14b)
[0148] 8a in Step 2i in Example 1 was replaced with 8b for reaction. Yield 77.5%, HRMS (ESI): [M+H] + 427.1683.
[0149] The confirmed data of the compound structure are:
[0150] 1 H NMR (400MHz,DMSO)δ10.58(s,1H),10.27(s,1H),9.49(s,1H),8.68(s,1H),8.40(s,1H),7.60(d,J= 8Hz, 2H), 7.51(d, J=8Hz, 1H), 7.39(d, J=8Hz, 1H), 7.34-7.28(m, 3H), 7.12(d, J=8Hz, 1H), 7.06(d , J=8Hz, 2H), 7.01(d, J=8Hz, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com