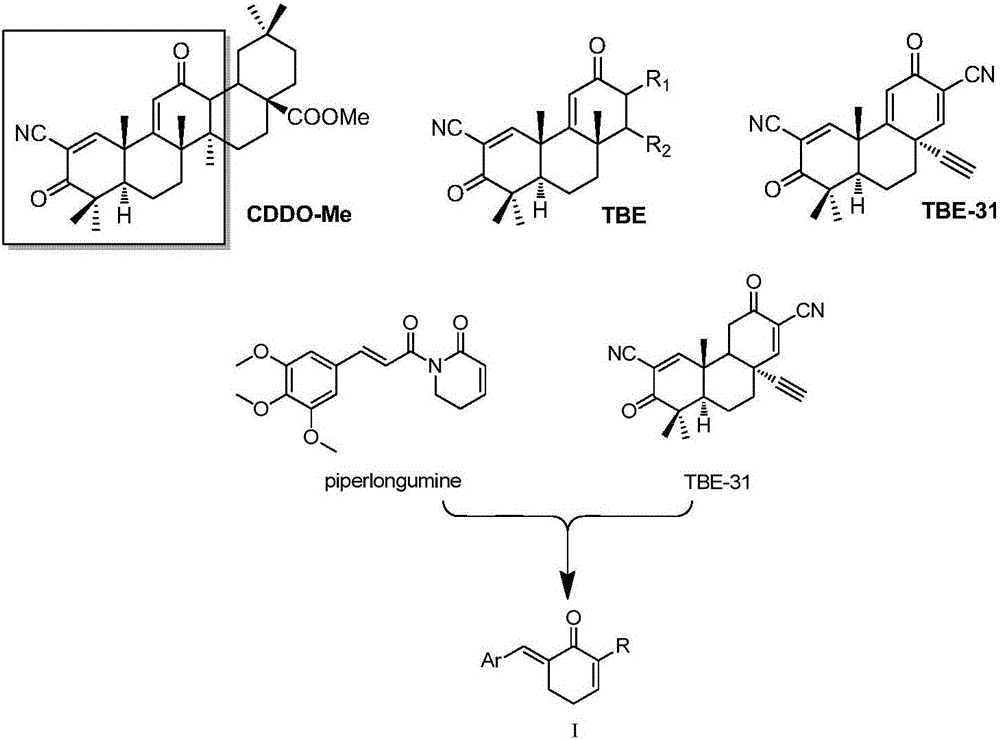
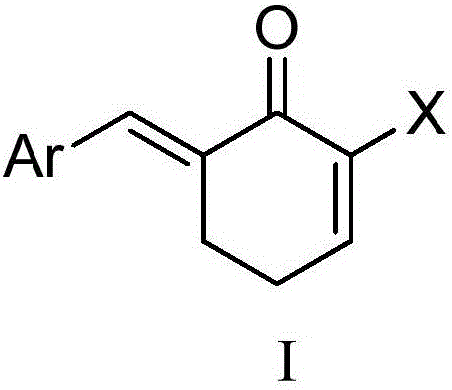
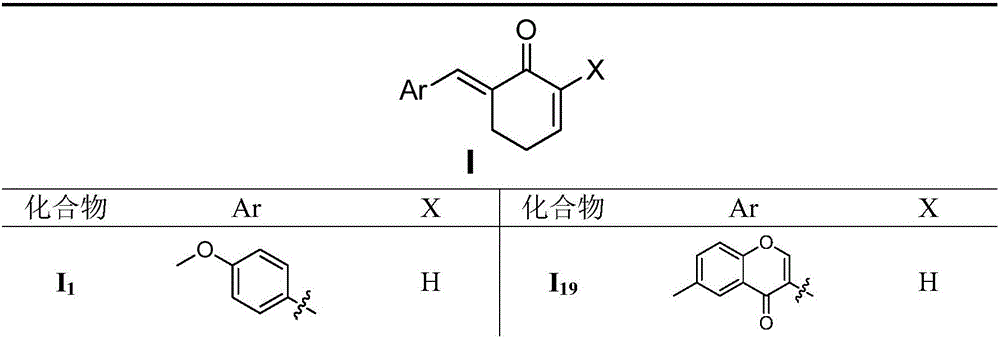
Benzyl cyclohexenone derivative and preparation method and medical application thereof
A technology of cyclohexenone and benzylidene, applied in the preparation of carbon-based compounds, the preparation of cyanide reactions, the preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1 (E)-6-(4-methoxyphenylvinylidene) cyclohexen-2-ketone (I 1 )
[0079] Dissolve the raw material cyclohexen-2-one (0.95g, 10.0mmol) in 15ml of anhydrous dichloromethane, and then add 200mg of TiCl at -50°C 4 and PPh 3 (2.62g, 10.0mmol), 4-methoxybenzaldehyde (2.72g, 20.0mmol) was added after 15min, and reacted overnight at room temperature. Add 10% K after the reaction is complete 2 CO 3 The solution was treated for 10 min, the organic layer was collected, rotary evaporated, and purified by column chromatography. 1.69 g of solid were obtained, yield: 79%. 1 H NMR (DMSO-d 6 ,300MHz): δ7.54(s,1H,ArCH=C),7.31(d,2H,J=0.60Hz Ar-H),6.97(dt,1H,CH=C H CO),6.89(m,1H,Ar-H),6.18(dt,1H,C H =CHCO),3.80(s,3H,OCH 3 ),2.99(m,2H,CH 2 ),2.38(m,2H,CH 2 ).MS(ESI)m / z:215[M+H] + .
Embodiment 2
[0080] Embodiment 2 (E)-6-(3-methoxyphenylvinylidene) cyclohexen-2-ketone (I 2 )
[0081] Dissolve the raw material cyclohexen-2-one (0.95g, 10.0mmol) in 15ml of anhydrous dichloromethane, and then add 200mg of TiCl at -50°C 4 and PPh 3 (2.62g, 10.0mmol), 3-methoxybenzaldehyde (2.72g, 20.0mmol) was added after 15min, and reacted overnight at room temperature. Add 10% K after the reaction is complete 2 CO 3 The solution was treated for 10 min, the organic layer was collected, rotary evaporated, and purified by column chromatography. 1.71 g of solid were obtained, yield: 80%. 1 H NMR (DMSO-d 6 ,300MHz):δ7.54(s,1H,ArCH=C),7.27(m,1H,Ar-H),7.00(m,1H,Ar-H),6.93(d,1H,CH=C H CO),6.84(m,1H,Ar-H),6.20(m,1H,C H =CHCO),3.79(s,3H,OCH 3 ),2.99(m,2H,CH 2 ),2.39(m,2H,CH 2 ).MS(ESI)m / z:215[M+H] + .
Embodiment 3
[0082] Example 3 (E)-6-(4-(trifluoromethoxy) phenylvinylidene) cyclohexen-2-one (I 3 )
[0083] Dissolve the raw material cyclohexen-2-one (0.95g, 10.0mmol) in 15ml of anhydrous dichloromethane, and then add 200mg of TiCl at -50°C 4 and PPh 3 (2.62g, 10.0mmol), 4-trifluoromethoxybenzaldehyde (3.80g, 20.0mmol) was added after 15min, and reacted overnight at room temperature. Add 10% K after the reaction is complete 2 CO 3 The solution was treated for 10 min, the organic layer was collected, rotary evaporated, and purified by column chromatography. 2.25 g of solid were obtained, yield: 84%. 1 H NMR (DMSO-d 6 ,300MHz):δ7.49(s,1H,ArCH=C),7.32(m,2H,Ar-H),7.17(m,2H,Ar-H),6.98(dt,1H,CH=C H CO),6.17(dt,1H,C H =CHCO),2.92(m,2H,CH 2 ),2.36(m,2H,CH 2 ).MS(ESI)m / z:269[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com