Application of heart-invigorating and calming tablet in preparation of medicine for curing heart diseases
A heart disease, Yixinning technology, used in cardiovascular system diseases, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0020] 1. Related materials
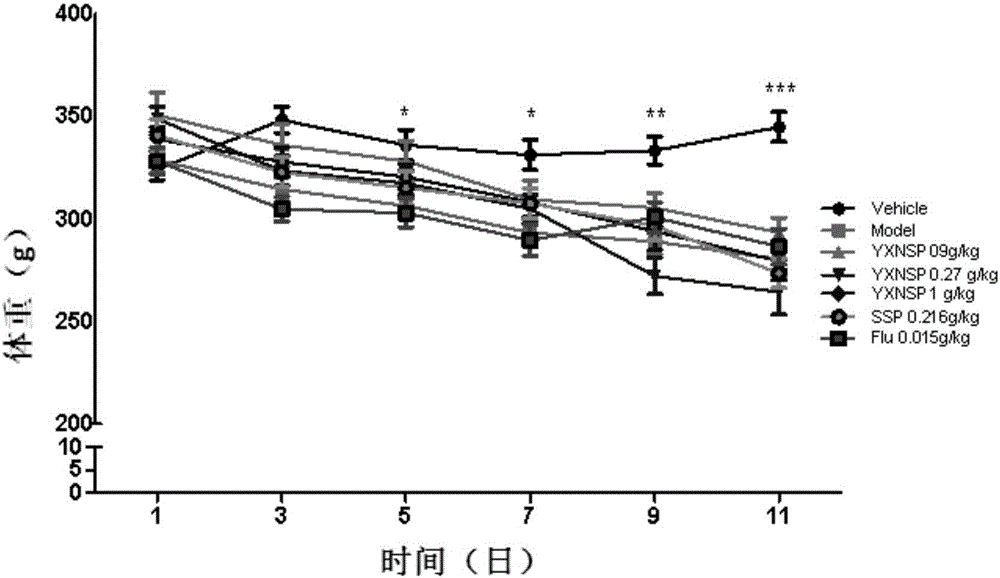
[0021] From June 27 to July 30, 2016, 98 male SD rats, weighing 220-260g, were selected from the Experimental Animal Center of Shenyang Pharmaceutical University, certificate number: SCXK (Liao) 2015-001. During the adaptation period (3d), the animals were kept in an environment with a light-dark cycle (light on 8:00) at a room temperature of 23±2°C, with free access to food and water. The rats were acclimatized for 3 days after being led, and administered continuously for 21 days. On the 22nd, the sleep deprivation model was started, and the modeling time was 10 days. Yixin Ningshen Tablets were continued to be given for treatment at the same time. On the 6th day of modeling and drug administration, the new object discrimination experiment was tested, on the 7th day, the elevated plus maze was tested, on the 8th day, the Y maze was tested, on the 9th day, the open field activity was tested, and on the 10th and 11th day, the electrocardiogram, orb...
experiment example 2
[0122] 1. Experimental time: July-August 2016.
[0123] 2. Experimental animals: SD rats, 75 males, weighing 160-180g, provided by the Experimental Animal Center of Shenyang Pharmaceutical University, certificate number: SCXK (Liao) 2015-001. During the adaptation period (3d), the animals were kept in an environment with a light-dark cycle (light on 8:00) at a room temperature of 23±2°C, with free access to food and water.
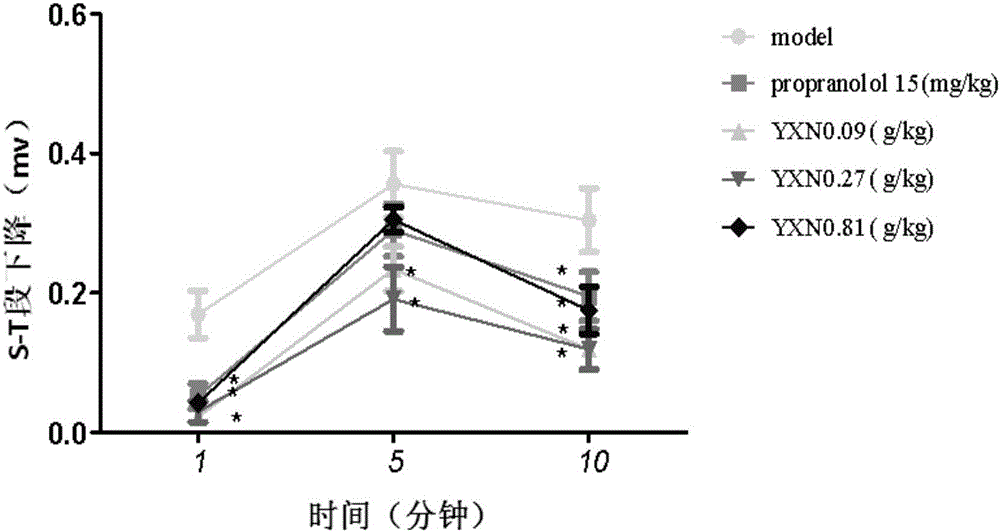
[0124] 3. Experimental grouping: 75 male SD rats, divided into 5 groups according to body weight, 15 in each group; model group, Yixin Ningshen Tablet 1 / 3×clinical equivalent (0.09g / kg), Yixin Ningshen Tablet 1× clinical equivalent dose (0.27g / kg), Yixin Ningshen Tablet 3× clinical equivalent dose (0.81g / kg), propranolol 1× clinical equivalent dose (15mg / kg) group . The specific experimental groups are shown in Table 12.
[0125] Table 12: Experimental grouping
[0126]
[0127] 4. Experimental method
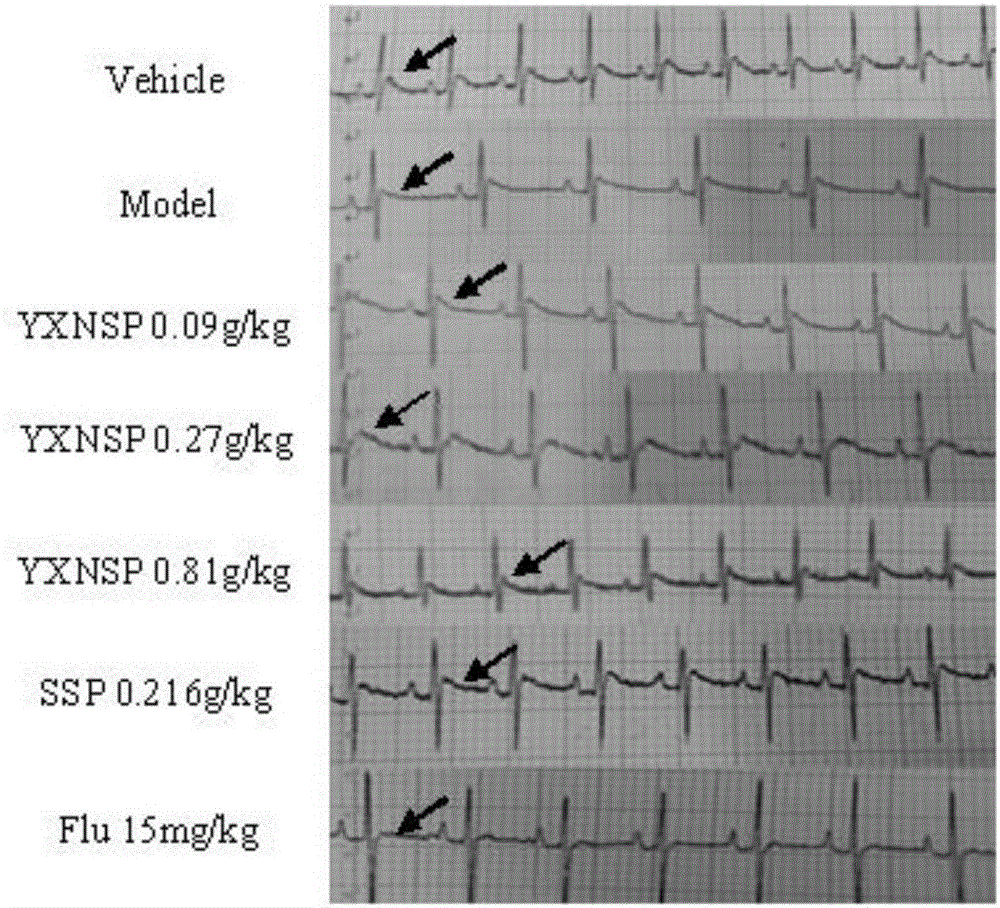
[0128] 1) Electrocardiogram detection
[012...
experiment example 3
[0145] 1. Clinical data
[0146] From May 2015 to May 2016, 120 patients met the inclusion criteria in the outpatient and inpatient departments of the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine. They were divided into the observation group and the control group according to the order of visit time by computer, with 60 people in each group.
[0147] 2. Diagnostic criteria:
[0148] 1) Diagnostic criteria for coronary heart disease:
[0149] Refer to the diagnostic criteria for coronary heart disease in the Guidelines for the Diagnosis and Treatment of Chronic Stable Angina Pectoris published by the Chinese Medical Association Cardiovascular Branch and the Editorial Committee of the Chinese Journal of Cardiovascular Diseases in 2007.
[0150] 2) Diagnostic criteria for anxiety and depression:
[0151] Refer to the "Chinese Mental Disorders Classification and Diagnostic Criteria Third Edition" issued by the Chinese Journal of Psychiatry in 2001 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com