A large specific surface porous zirconia mesoscopic crystal and its preparation method and application
A zirconia mesoscopic and large specific surface technology, applied in zirconia, chemical instruments and methods, catalyst activation/preparation, etc., to achieve the effects of high yield, abundant pores, and excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
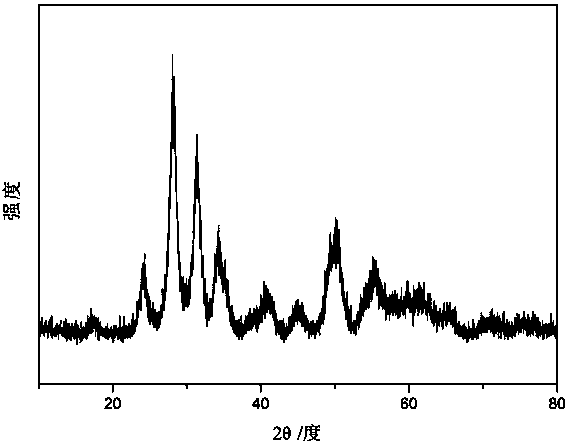
[0023] Dissolve 3.50 g of polyethylene glycol 2000, 11.28 g of zirconium oxychloride octahydrate and 4.20 g of urea in 50 mL of deionized water to prepare a mixed solution. The above mixed solution was calibrated to 70 mL with deionized water and transferred to a volume of In a 100 mL high-temperature reactor (that is, the molar concentration of zirconium oxychloride octahydrate is 0.5 mol / L, the amount of polyethylene glycol 2000 added is 0.05 g per milliliter of mixed solution, and the molar ratio of zirconium oxychloride octahydrate to urea is 1:2). The reaction kettle was put into a blast drying oven, the reaction temperature was controlled at 150 °C, and the reaction time was 6 h. The obtained product was washed by centrifugation to remove impurity ions, and then dried at 60°C for 8 h to obtain mesoscopic crystals of zirconia.
[0024] figure 1 It is the XRD figure of the zirconia mesoscopic crystal prepared in the present embodiment. Depend on figure 1 It can be seen...
Embodiment 2
[0029] Dissolve 7.00 g of polyethylene glycol 2000, 11.28 g of zirconium oxychloride octahydrate and 4.20 g of urea in 50 mL of deionized water to prepare a mixed solution. The above mixed solution is calibrated to 70 mL with deionized water and transferred to a volume of In a 100 mL high-temperature reactor (that is, the molar concentration of zirconium oxychloride octahydrate is 0.5 mol / L, the amount of polyethylene glycol 2000 added is 0.1 g per milliliter of mixed solution, and the molar ratio of zirconium oxychloride octahydrate to urea is 1:2). The reaction kettle was put into a blast drying oven, the reaction temperature was controlled at 150 °C, and the reaction time was 6 h. The obtained product was washed by centrifugation to remove impurity ions, and then dried at 60°C for 8 h to obtain mesoscopic crystals of zirconia.
[0030] Figure 5 It is the SEM figure of the zirconia mesoscopic crystal prepared in the present embodiment. Figure 5 It shows that the prepare...
Embodiment 3
[0033] 14.00 g of polyethylene glycol 2000, 11.28 g of zirconium oxychloride octahydrate and 4.20 g of urea were dissolved in 50 mL of deionized water to prepare a mixed solution, and the above mixed solution was calibrated to 70 mL with deionized water and transferred to a volume of In a 100 mL high-temperature reactor (that is, the molar concentration of zirconium oxychloride octahydrate is 0.5 mol / L, the amount of polyethylene glycol 2000 added is 0.2 g per milliliter of mixed solution, and the molar ratio of zirconium oxychloride octahydrate to urea is 1:2). The reaction kettle was put into a blast drying oven, the reaction temperature was controlled at 150 °C, and the reaction time was 6 h. The obtained product was washed by centrifugation to remove impurity ions, and then dried at 60°C for 8 h to obtain mesoscopic crystals of zirconia.
[0034] Figure 6 It is the SEM figure of the zirconia mesoscopic crystal prepared in the present embodiment. Figure 6 It shows that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com