Application of compound HUBIN-1 in preparation of drug for preventing and/or treating liver inflammatory diseases
A technology for HUBIN-1 and inflammatory diseases, applied in the field of drug research and development, can solve problems such as not being able to be used on a large scale, and achieve obvious social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of compound HUBIN-1 solution
[0040] Compound HUBIN-1, the stock solution of compound HUBIN-1 prepared after DMSO solubilization, was diluted with PBS to prepare the solvent of compound HUBIN-1 before injection.
Embodiment 2
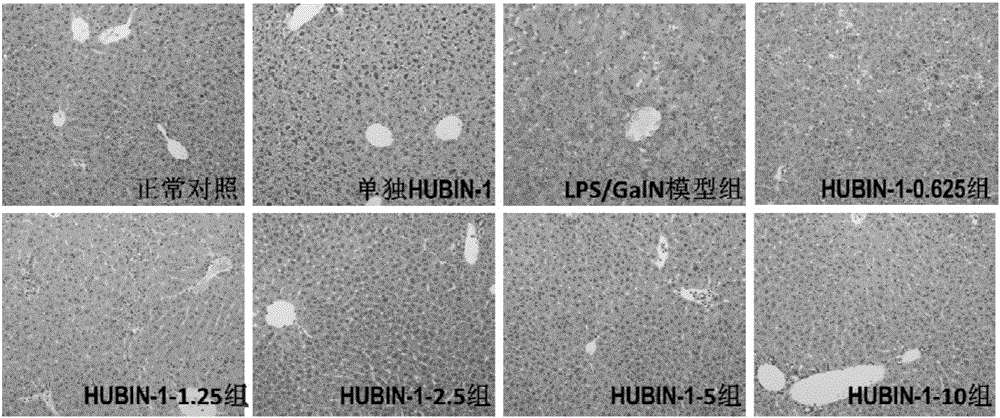
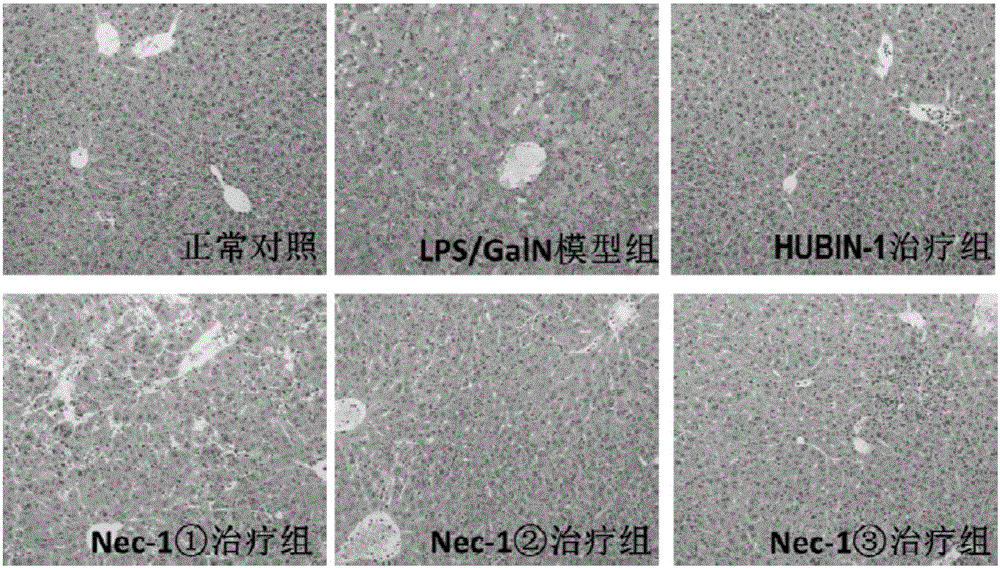
[0041] Example 2 The protective effect of compound HUBIN-1 on LPS / D-GalN-induced hepatitis in mice-dose-effect relationship study
[0042] Animal models are research tools to verify whether new therapeutic drugs and new treatment methods are effective. At present, the animal models for simulating severe hepatitis used in the world include drug-induced severe hepatitis models (paracetamol model, LPS / D-GalN model, etc.) , thioacetamide model, carbon tetrachloride model, etc.), acute liver ischemia model, partial hepatectomy model, etc. Among them, the LPS / D-GalN model is an ideal severe hepatitis animal model because of its good reproducibility, non-obvious extrahepatic toxicity, and clinical liver damage.
[0043] Six-week-old male C57BL / 6 mice (purchased from Nanjing Institute of Biomedicine) were randomly divided into normal control group, compound HUBIN-1 group, model group (LPS / D-GalN group), treatment group (0.625mg / kg HUBIN -1+LPS / D-GalN group, 1.25mg / kg HUBIN-1+LPS / D-Ga...
Embodiment 3
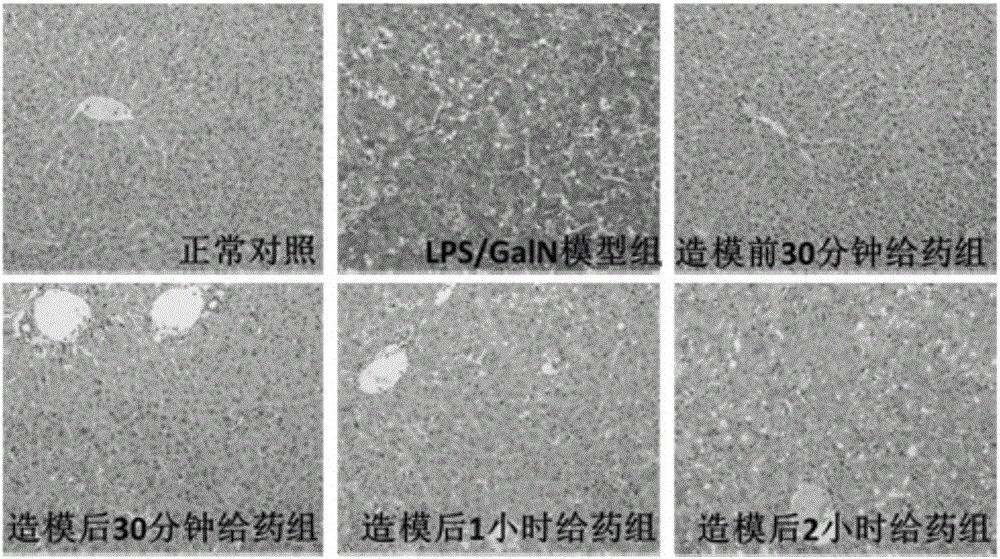
[0050] Example 3 Therapeutic effect of compound HUBIN-1 on LPS / D-GalN-induced hepatitis in mice-time-effect relationship study
[0051] Six-week-old male C57BL / 6 mice (purchased from Nanjing Institute of Biomedicine) were randomly divided into normal control group, model group (LPS / D-GalN group), treatment group (2.5mg / kg HUBIN-1+LPS / D -GalN group). The treatment group was intraperitoneally injected with LPS / D-GalN (10 μg / kg LPS + 400 mg / kg D-GalN), and administered 30 minutes before LPS / D-GalN modeling, 30 minutes, 1 hour, and 2 hours after modeling. The compound HUBIN-1 was injected intraperitoneally at a dose of 2.5 mg / kg; the model group was replaced with an equal volume of solvent for the compound HUBIN-1; the normal control group was not treated. Six hours after the LPS / D-GalN model was established, the blood was collected by removing the eyeball, and the liver tissue was fixed and frozen. Biochemical and pathological examinations were performed to evaluate the degree o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com