Catalyst for producing ethylbenzene from benzene and ethane
A catalyst and ethane production technology, which is applied in molecular sieve catalysts, physical/chemical process catalysts, organic chemistry, etc., and can solve the problems of low conversion rates of benzene and ethane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Catalyst preparation
[0046] Weigh chloroplatinic acid containing 0.5g Pt (molecular formula is H 2 PtCl 6 ·6H 2 O), zinc nitrate equivalent to 0.7g ZnO (molecular formula is Zn(NO 3 ) 2 ·6H 2 O) and equivalent to 0.4g CeO 2 Cerium nitrate (molecular formula is Ce(NO 3 ) 3 ·6H 2O) Add it into 100mL deionized water, stir to make it all dissolve, and form a mixed solution; weigh 98.4g of hydrogen-type ZSM-5 molecular sieve (Si / Al molar ratio is 46), then add it to the above mixed solution, and soak for 3h , Dry at 100°C for 12h, and bake at 500°C for 2h.
[0047] The above product was tabletted and pulverized to 20-40 meshes, and a hydrogen-nitrogen mixed gas with a hydrogen volume content of 5% was used as a reducing agent, the reduction temperature was 350°C, and the reduction time was 1 hour to obtain a catalyst. The catalytic composition is shown in Table 1.
[0048] 2. Catalyst evaluation
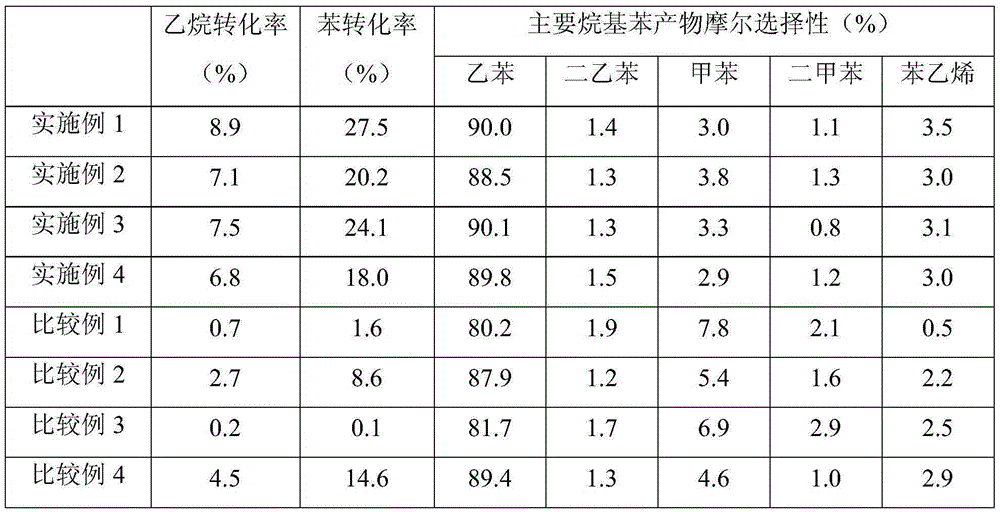
[0049] The reaction of benzene and ethane to ethylbenzene is c...
Embodiment 2
[0051] 1. Catalyst preparation
[0052] Weigh chloroplatinic acid containing 0.5g Pt (molecular formula is H 2 PtCl 6 ·6H 2 O), zinc nitrate equivalent to 0.4g ZnO (molecular formula is Zn(NO 3 ) 2 ·6H 2 O) and equivalent to 0.7g CeO 2 Cerium nitrate (molecular formula is Ce(NO 3 ) 3 ·6H 2 O) Add it into 100mL deionized water, stir to make it all dissolve, and form a mixed solution; weigh 98.4g of hydrogen-type ZSM-5 molecular sieve (Si / Al molar ratio is 46), then add it to the above mixed solution, and soak for 3h , Dry at 100°C for 12h, and bake at 500°C for 2h.
[0053] The above product was tabletted and pulverized to 20-40 meshes, and a hydrogen-nitrogen mixed gas with a hydrogen volume content of 5% was used as a reducing agent, the reduction temperature was 350°C, and the reduction time was 1 hour to obtain a catalyst. The catalytic composition is shown in Table 1.
[0054] 2. Catalyst evaluation
[0055] The same method as in Example 1 was used to evaluate t...
Embodiment 3
[0057] 1. Catalyst preparation
[0058] Weigh chloroplatinic acid containing 0.5g Pt (molecular formula is H 2 PtCl 6 ·6H 2 O), zinc nitrate equivalent to 0.7g ZnO (molecular formula is Zn(NO 3 ) 2 ·6H 2 O) and equivalent to 0.4g La 2 o 3 Lanthanum nitrate (molecular formula La(NO 3 ) 3 ·6H 2 O) Add it into 100mL deionized water, stir to make it all dissolve, and form a mixed solution; weigh 98.4g of hydrogen-type ZSM-5 molecular sieve (Si / Al molar ratio is 46), then add it to the above mixed solution, and soak for 3h , Dry at 100°C for 12h, and bake at 500°C for 2h.
[0059] The above product was tabletted and pulverized to 20-40 meshes, and a hydrogen-nitrogen mixed gas with a hydrogen volume content of 5% was used as a reducing agent, the reduction temperature was 350°C, and the reduction time was 1 hour to obtain a catalyst. The catalytic composition is shown in Table 1.
[0060] 2. Catalyst evaluation
[0061] The same method as in Example 1 was used to evalua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com