Cloning of novel glutamate decarboxylase gene and application thereof
A technology of glutamic acid decarboxylase and glutamic acid, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of pH dropping to 4.5 or below, large equipment loss, low activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The specific implementation method of the present invention is illustrated below through specific examples, but these examples do not constitute a limitation to the mode, scope and effect of the present invention.
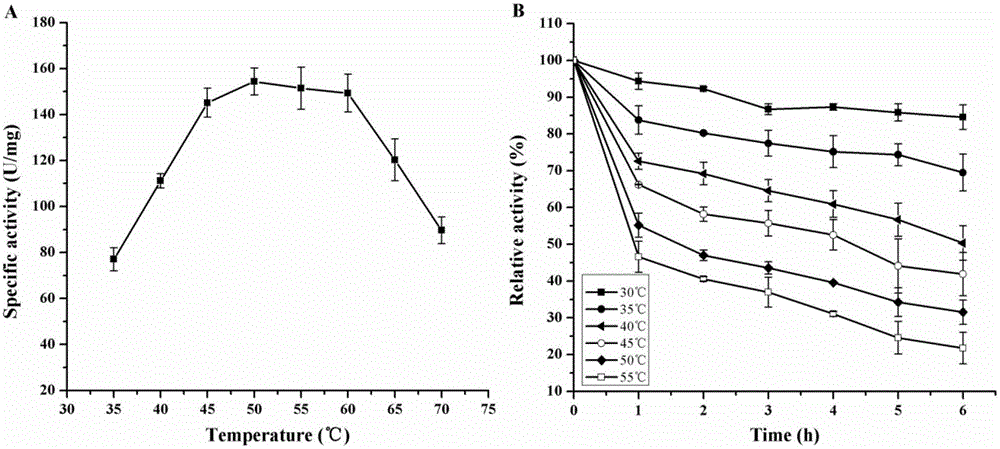
[0022] The glutamic acid decarboxylase gene was obtained from the China Microorganism Culture Collection and Management Center, and the strain number was 10055. There are 1404 nucleotide sequences, and the encoded protein size is 53 kDa. Through sequence comparison, it was found that the glutamic acid decarboxylase gene of Bacillus megaterium was about 50% and 30% similar to the glutamic acid decarboxylase gene of Escherichia coli and lactic acid bacteria, respectively.
[0023] Construction of recombinant strains
[0024] PCR primers were designed based on the glutamic acid decarboxylase gene sequence of the Bacillus megaterium model strain, and the BmGAD gene fragment was obtained by PCR using the Bacillus megaterium genome as a template. Recombinant pla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com