Polypeptide for inhibiting HIV infection and medicinal application thereof
A technology of derivatives and stereoisomers, applied in medical preparations containing active ingredients, peptide sources, applications, etc., to achieve the effects of enhancing antiviral activity, saving synthesis costs, and simplifying sequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
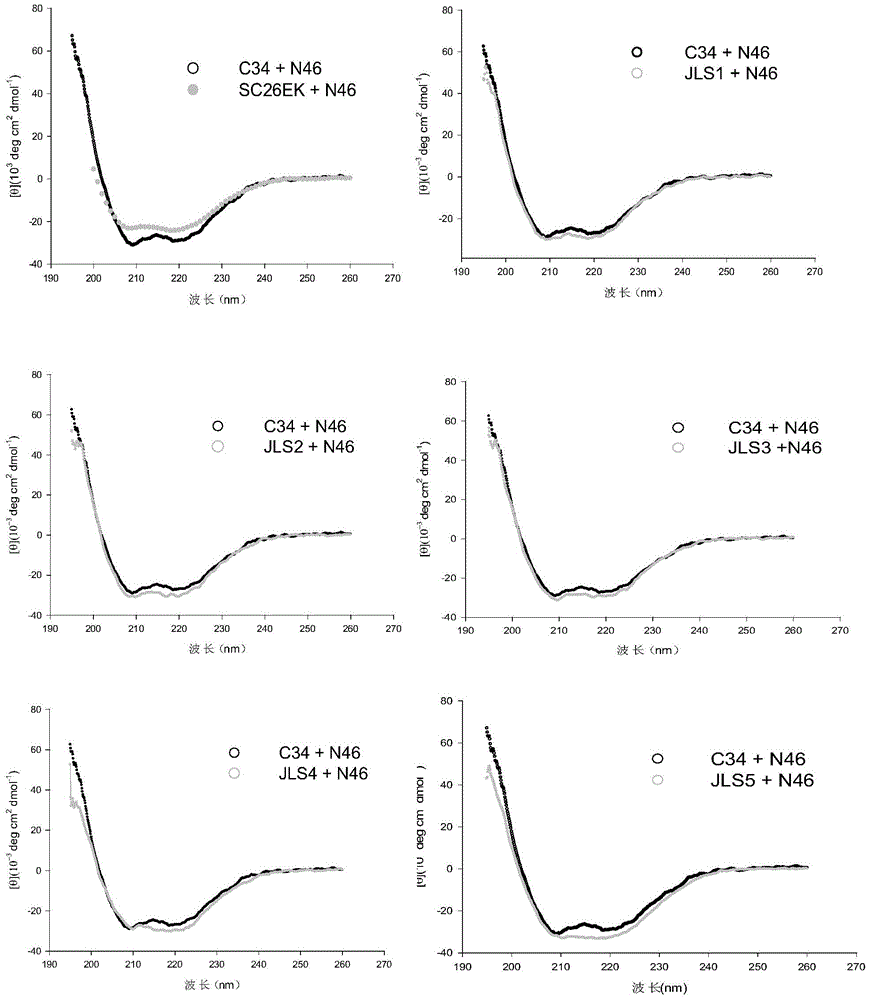
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of polypeptide
[0052] Synthetic polypeptide JLS1-JSL5 (in this example, synthesized by SynPeptide Co Ltd), was purified and identified by high performance liquid chromatography (HPLC) and mass spectrometry (PerSeptive Biosystems, Framingham, MA), showing that the sequence was correct and the purity was greater than 98%; molecular weight and purity of polypeptide of the present invention are as shown in table 1
[0053] Table 1. Molecular weight and purity of peptides
[0054]
[0055]
Embodiment 2
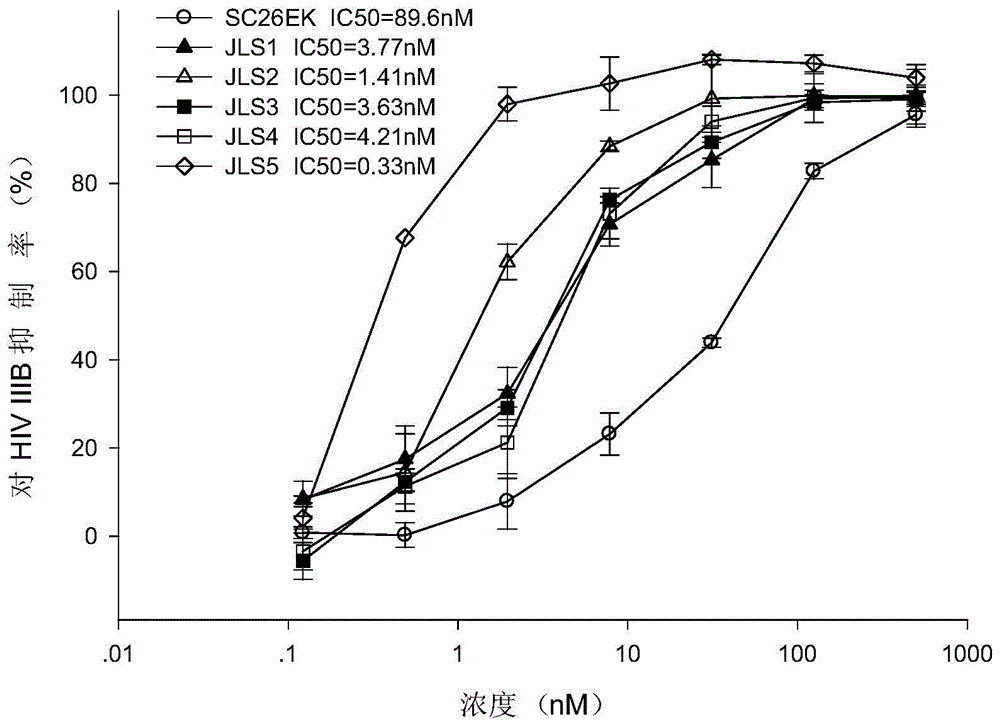
[0056] Example 2: Polypeptide inhibits HIV-infected cells
[0057] 2.1 Experimental materials and methods
[0058] 2.1.1 The method for detecting the activity of C polypeptide in inhibiting HIV-1X4 strain IIIB infection of MT-2 cells is as follows: add 50 μL of IIIB virus (diluted with serum-free 1640) and 50 μL of doubling-diluted (4-fold) to each well of a 96-well plate C polypeptide (diluted with serum-free 1640), incubated at 37°C for 30 minutes, then added 100 μL of cells (10 5 cells / ml, diluted with 1640 culture medium with 10% serum), the wells that only added the same final concentration of MT-2 cells were used as negative controls, and the wells that added the same final concentration of IIIB virus and MT-2 cells were not used. The well where the drug was added was the positive control; on the second day, 150 μL of the supernatant was aspirated from each well, and 150 μL of fresh 1640 culture solution containing 10% serum was added; on the fifth day, 50 μL of the sup...
Embodiment 3
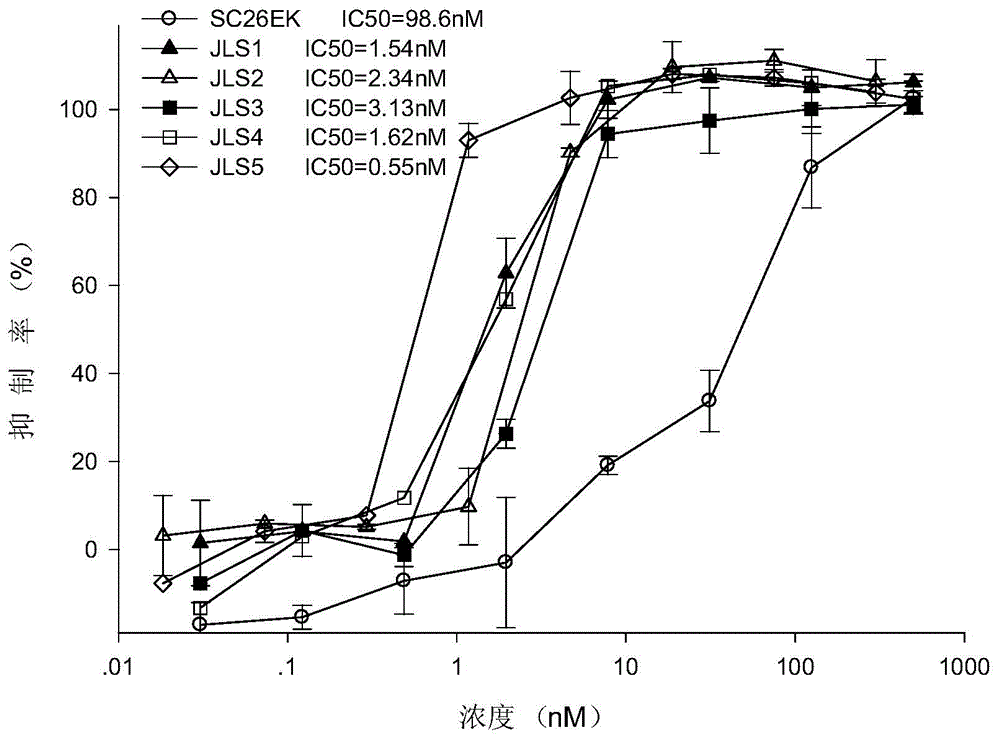
[0063] Example 3. Broad-spectrum anti-HIV effect of JLS2 and JLS5
[0064] The retrovirus of HIV is prone to genome mutations, the present invention selects JSL2 and JSL5, which have better effects on laboratory-adapted strains, and detects their antiviral activity to a series of clinical strains; clinical strains can be divided into many subtypes, including A-D, F-H, J and K subtypes, etc. In this experiment, SC26EK was used as a control to observe the effect of its N-terminal and C-terminal plus enhanced sequences. At the same time, T20, the only fusion inhibitor currently on the market, and The fusion inhibitor HP23 with the highest antiviral activity reported now is used as a positive control;
[0065] The method is as follows: add 50 μL of HIV virus (diluted with serum-free 1640) and 50 μL of double-diluted (4-fold) C polypeptide (diluted with serum-free 1640) to each well of a 96-well plate, incubate at 37°C for 30 minutes, and then add 100 μL cells (10 5cells / ml, dilu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com