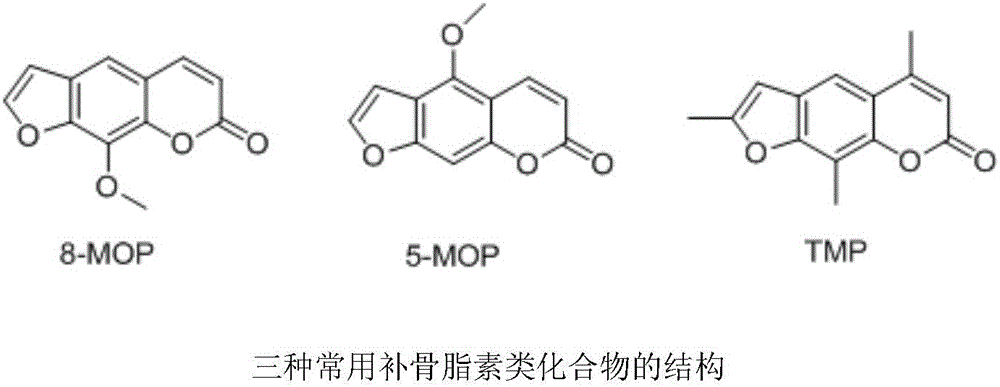
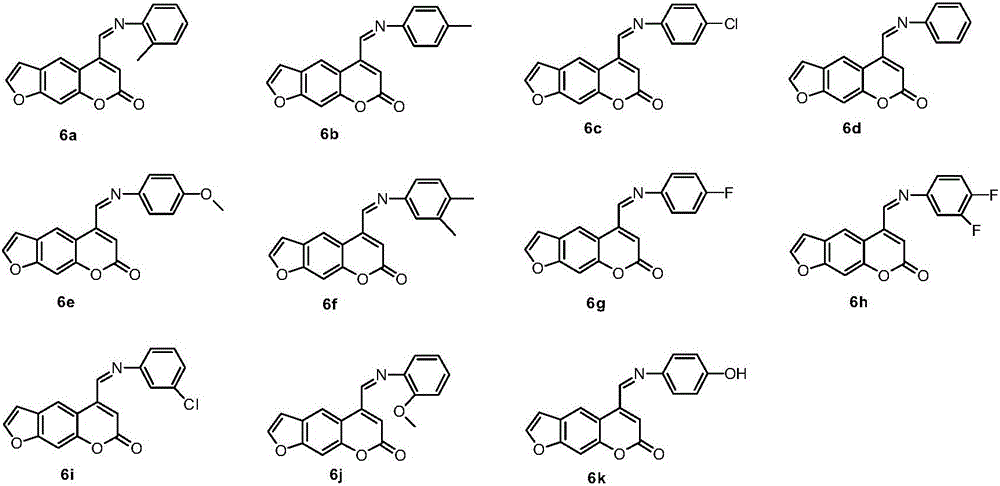
Psoralen schiff base derivatives and application
A technology of Schiff bases and psoralen is applied in the directions of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc. Problems such as weak research on material basis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Preparation of Intermediate 2:
[0079] At room temperature, dissolve 1.76g (10mmol) of intermediate 1 and 2.76g (20mmol) of potassium carbonate in 50mL of acetone, then add 1.20g (15mmol) of 2-chloroethanol, heat to reflux and react until all the raw materials disappear , stop the reaction, cool to room temperature, filter, the filtrate is concentrated, the residue is eluted by column chromatography gradient, the eluent is petroleum ether: ethyl acetate with a volume ratio of 1:1, and 2.10g of intermediate 2,4- Methyl-7-hydroxyethoxycoumarin;
[0080] NMR data of 4-methyl-7-hydroxyethoxycoumarin (intermediate 2):
[0081] 1 H NMR (400MHz, CDCl 3 )d 7.51(d, J=9.0Hz, 1H), 6.91–6.83(m, 2H), 6.15(d, J=1.1Hz, 1H), 4.15(t, J=8.7Hz, 2H), 4.01(m ,2H),2.40(d,J=1.1Hz,3H);
[0082] Preparation of intermediate 3:
[0083] Under low-temperature reaction conditions of -78°C, dissolve 50.25g (2mmol) oxalyl chloride in a small amount of dry dichloromethane, and slowly add dropwi...
Embodiment 2
[0100] Preparation of compound 6b:
[0101] Compound 2-compound 5 were prepared according to Example 1:
[0102] Dissolve 0.21g (1mmol) of compound 5 and 0.16g (1.5mmol) of 4-methylaniline in absolute ethanol, reflux the reaction until all the raw materials disappear, cool to room temperature, concentrate the mother liquor, and elute the residue by column chromatography gradient , the eluent is petroleum ether:ethyl acetate with a volume ratio of 15:1, to obtain 0.11g of compound 6b, (Z)-5-(4-methylphenylimine)methyl-7H-fur[3, 2-g] benzopyran-7-one;
[0103] NMR data of (Z)-5-(4-methylphenylimine)methyl-7H-furo[3,2-g]benzopyran-7-one (compound 6b):
[0104] 1 H NMR (400MHz, CDCl 3 )δ9.18(s,1H),8.67(s,1H),7.70(d,J=2.2Hz,1H),7.54(s,1H),7.05(d,J=8.1Hz,2H),6.90– 6.85(m,3H),6.77(s,1H),2.42(s,3H).
[0105] 13 C NMR (151MHz, CDCl 3 )δ 161.19, 156.35, 152.60, 148.19, 147.01, 146.27, 138.29, 130.24, 125.14, 121.21, 119.60, 118.35, 116.04, 113.42, 107.14, 100.17, 21.31.
Embodiment 3
[0107] Preparation of compound 6c:
[0108] Compound 2-compound 5 were prepared according to Example 1:
[0109] Dissolve 0.21g (1mmol) of compound 5 and 0.19g (1.5mmol) of 4-chloroaniline in absolute ethanol, reflux the reaction until all the raw materials disappear, cool to room temperature, concentrate the mother liquor, and elute the residue by column chromatography gradient, The eluent is petroleum ether:ethyl acetate with a volume ratio of 15:1, and 0.09 g of compound 6c is obtained, and compound 6c is (Z)-5-(4-chlorophenylimine)methyl-7H-furan[3 ,2-g]benzopyran-7-one;
[0110] NMR data of (Z)-5-(4-chlorophenylimine)methyl-7H-furo[3,2-g]benzopyran-7-one (compound 6c):
[0111] 1 H NMR (600MHz, CDCl 3 )δ9.13(s,1H),8.64(s,1H),7.71(d,J=2.0Hz,1H),7.55(s,1H),7.45(d,J=7.4Hz,2H),7.28( d,J=8.2Hz,2H),6.89(d,J=1.9Hz,1H),6.78(s,1H).
[0112] 13 C NMR (151MHz, CDCl 3 )δ 160.84, 157.72, 147.23, 146.99, 145.68, 138.10, 133.61, 129.65, 125.06, 124.42, 122.36, 113.02, 119.28, 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com