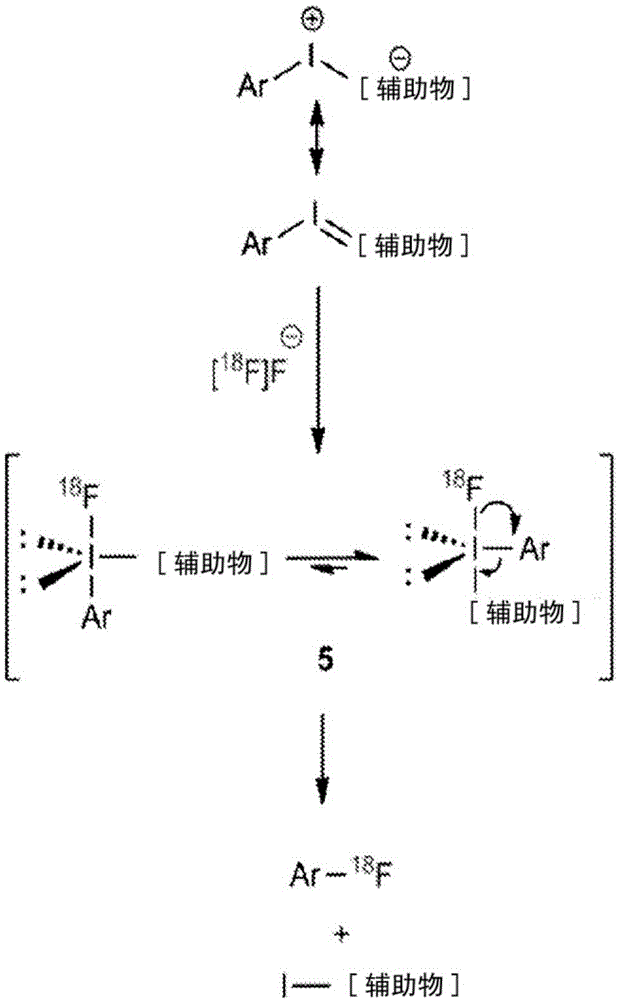
Iodine(III)-mediated radiofluorination
A technology of fluoride and iodonium, applied in the field of iodonium ylide compounds, to achieve the effects of easy refining, excellent regioselectivity, and easy implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0333] The preparation of the compounds of the present invention may involve the protection and deprotection of various chemical groups. One skilled in the art can readily determine the need for protection and deprotection and the selection of appropriate protecting groups. The chemistry of protecting groups is described, for example, in Kocienski, Protecting Groups, (Thieme, 2007); Robertson, Protecting Group Chemistry, (Oxford University Press, 2000); Smith et al., March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6 th Ed. (Wiley, 2007); Peturssion et al., "Protecting Groups in Carbohydrate Chemistry," J. Chem. Educ., 1997, 74(11), 1297; and Wuts et al., Protective Groups in Organic Synthesis, 4th Ed. , (Wiley, 2006).
[0334] The reaction can be monitored according to any suitable method known in the art. For example, product formation can be monitored by spectroscopic means, such as nuclear magnetic resonance spectroscopy (e.g., 1 H or 13 C), i...
Embodiment 1-27
[0593] Examples 1-27 were prepared according to General Method 8.
Embodiment 1
[0595] 1,3-Dimethylpyrimidine-2,4,6(1H,3H,5H)-trione-5-[1,1’-biphenyl-4-iodonium]ylide (8a)
[0596]
[0597] Yield: 32%, colorless solid. 1 H NMR (300MHz, CDCl 3 ): δ7.97 (d, J = 8.5Hz, 2H), 7.61 (d, J = 8.5Hz, 2H), 7.42–7.52 (m, 5H), 3.37 (s, 6H) ppm. 13 C NMR (75MHz, CDCl 3 ): δ161.1, 152.5, 145.2, 138.2, 137.3, 133.8, 130.1, 128.7, 126.8, 111.6, 68.3, 28.8ppm. HRMS(m / z): For C 18 h 16 IN 2 o 3 [M+H] of + Calculated, 435.0206; found 435.0192.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com