Anti-hepatitis C Daclatasvir synthesis method
A technology of anti-hepatitis C daclatasvir and synthetic method, which is applied in the field of synthesis of anti-hepatitis C daclatasvir, can solve the problems of complex synthetic route and high cost of production raw materials, and achieve simple reaction, low cost of production raw materials, and step-by-step synthetic route little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
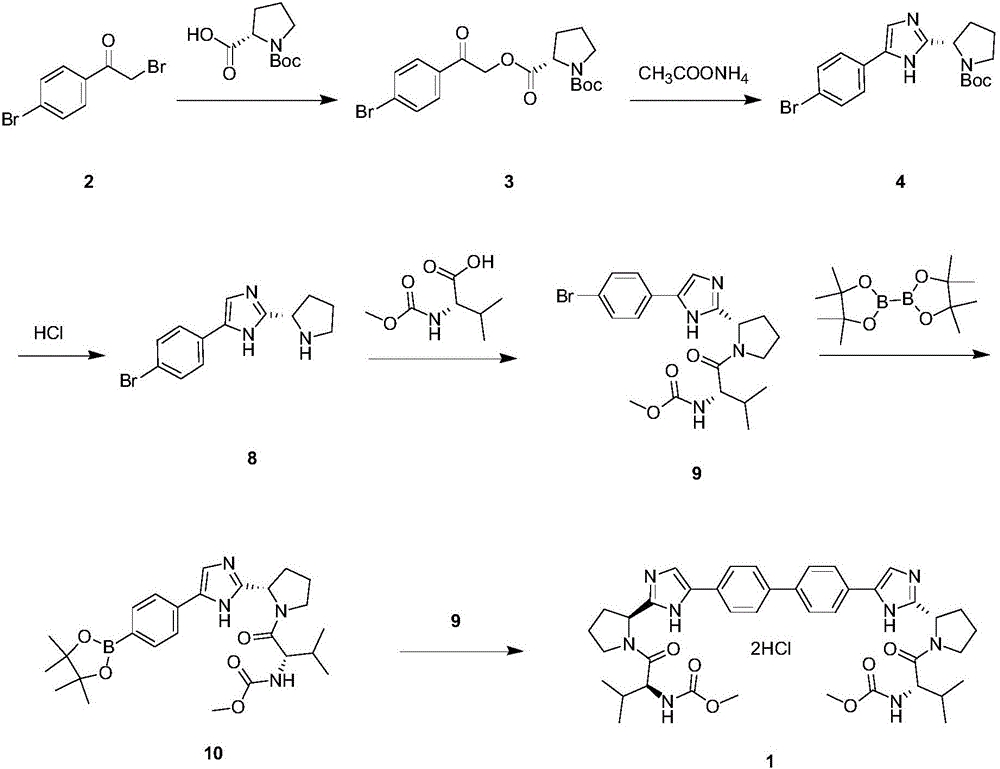
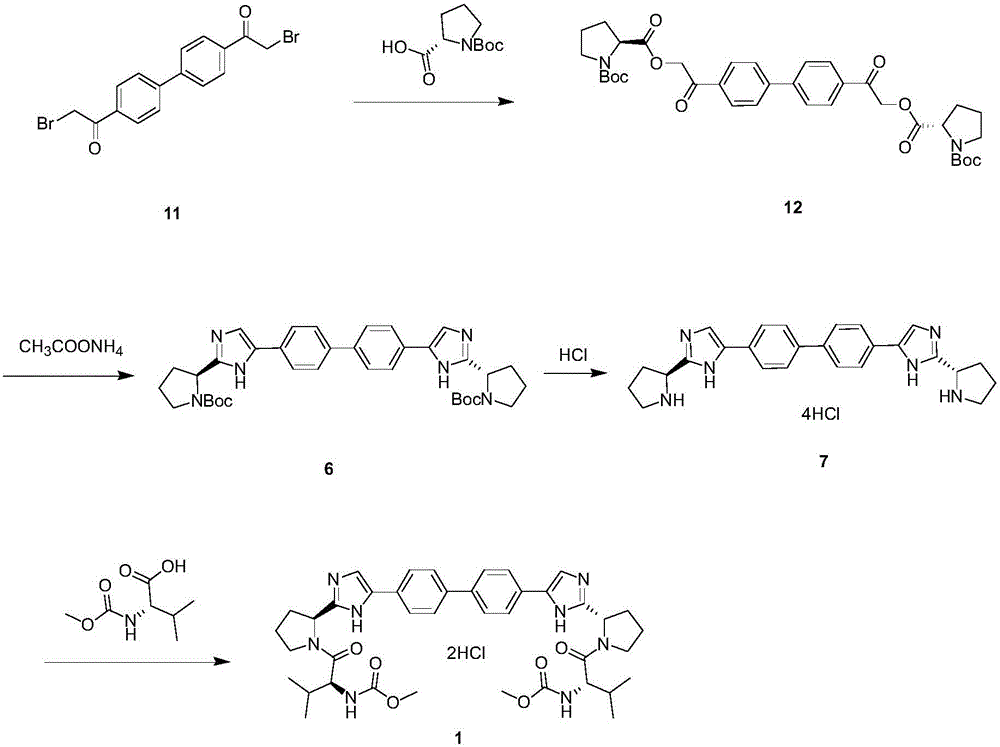
[0025] Such as Figure 4 Shown, a kind of synthetic method of anti-hepatitis C daclatasvir, this synthetic method comprises the following steps:
[0026] (1) Using 4,4'-bis(2-chloroacetyl)biphenyl to condense with Boc-L-proline to generate ester;
[0027] (2) take above-mentioned ester and ammonium acetate ring closure, obtain imidazole;
[0028] (3) De-Boc the imidazole to obtain the hydrochloride;
[0029] (4) Take the hydrochloride and carry out Moc-L-valine condensation to obtain daclatasvir.
specific Embodiment approach 1
[0031] (1) Synthesis of esters
[0032] Stage a. Put 4,4'-bis(2-chloroacetyl)biphenyl (7.5g, 24.4mmol), Boc-L-proline (10.5g, 48.8mmol) and acetonitrile (70mL) into the reaction flask , add diisopropylethylamine DIPEA (6.5g, 50mmol);
[0033] Stage b. Heat up to 25°C (temperature range is 25-82°C), stir for 36 hours, the reaction is completed, the reaction solution is concentrated under reduced pressure, and replaced with toluene solution (140mL);
[0034] Stage c, washing twice with saturated sodium chloride (70 mL), concentrating toluene to obtain an oily foamy solid, namely the desired ester (15.6 g, yield 96.3%).
[0035] (2) Synthesis of imidazole
[0036] Add ester (15.6g, 23.5mmol) and 95mL toluene to the reaction flask, add ammonium acetate (30g, 389mmol), heat up to 95°C (temperature range is 95-100°C), and react for 15 hours;
[0037] Cool down to 60°C, slowly add saturated aqueous sodium bicarbonate solution, adjust the pH to 7 (PH range 7-8);
[0038] Layer at ...
specific Embodiment approach 2
[0049] (1) Synthesis of esters
[0050] Stage a. Put 4,4'-bis(2-chloroacetyl)biphenyl (7.5g, 24.4mmol), Boc-L-proline (10.5g, 48.8mmol) and acetonitrile (70mL) into the reaction flask , add diisopropylethylamine DIPEA (6.5g, 50mmol);
[0051] Stage b: heat up to 65°C (temperature range is 25-82°C), stir for 8 hours, the reaction is completed, the reaction solution is concentrated under reduced pressure, and replaced with toluene solution (140mL);
[0052] Stage c, washing twice with saturated sodium chloride (70 mL), concentrating toluene to obtain an oily foamy solid, namely the desired ester (15.6 g, yield 96.3%).
[0053] (2) Synthesis of imidazole
[0054] Add ester (15.6g, 23.5mmol) and 95mL toluene to the reaction flask, add ammonium acetate (30g, 389mmol), heat up to 97°C (temperature range is 95-100°C), and react for 15 hours;
[0055] Cool down to 60°C, slowly add saturated aqueous sodium bicarbonate solution, and adjust the pH to 7.3 (pH range 7-8);
[0056] Laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com