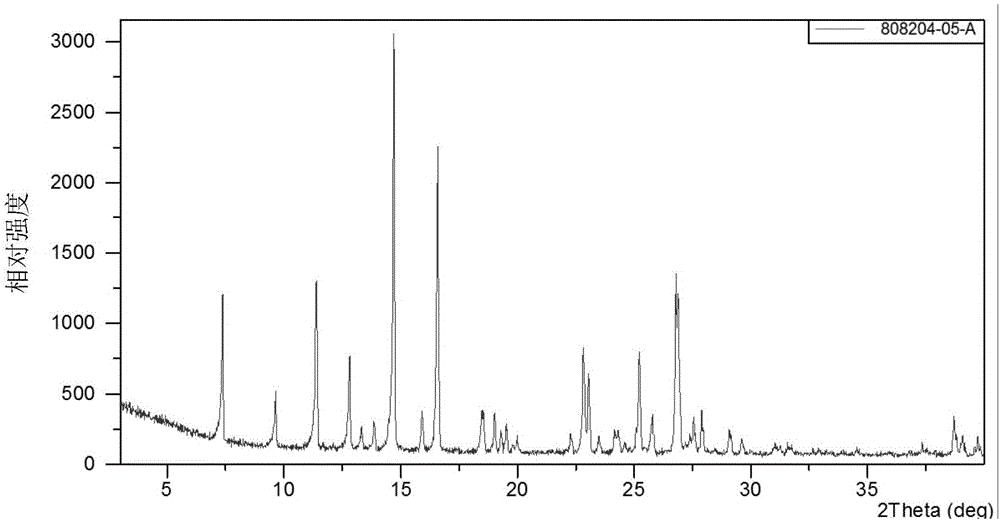
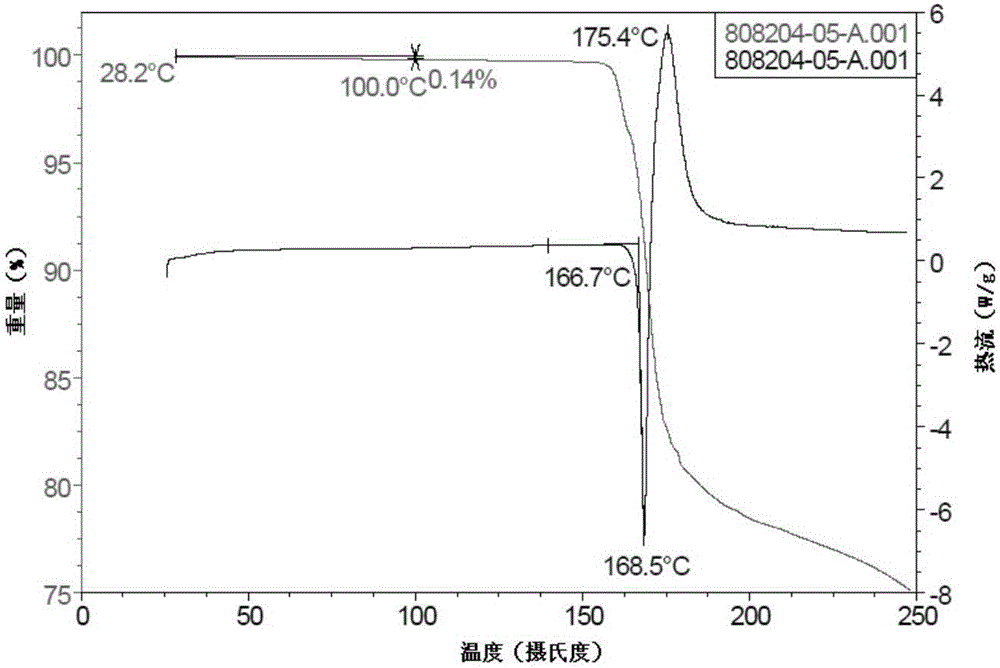
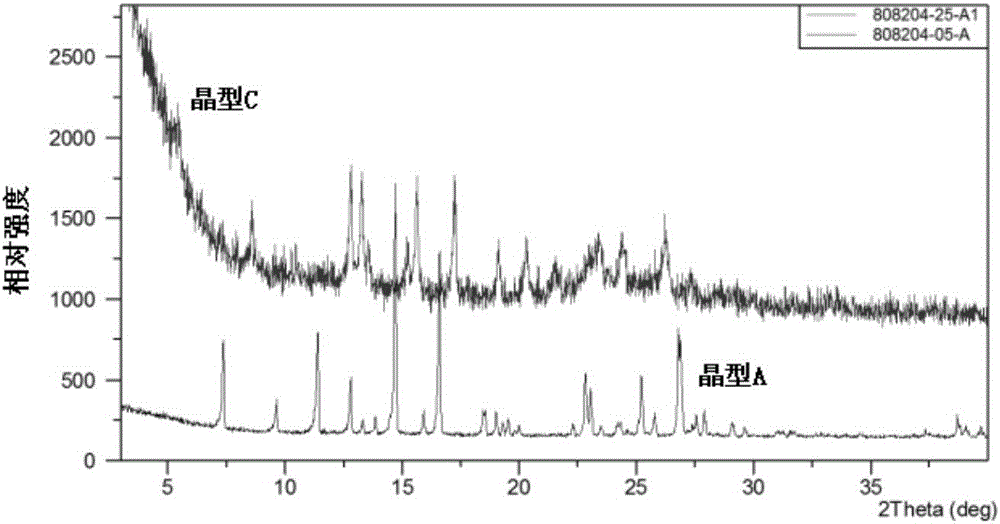
Crystal form A of gefitinib and method for preparing gefitinib
A kind of production technology and crystal form technology, which is applied in the field of biomedicine, can solve the problems of drug content, drug crystal form cannot be reproduced, low purity of the product, and poor method reproducibility, so as to achieve easy control of product quality, easy purchase, and environmental pollution. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: compound shown in preparation formula 2
[0092] Add 5kg of the compound shown in Formula 1, 55kg of acetic acid, and 50L of tetrahydrofuran into a 500L reactor, start stirring; add 6kg of iron powder, heat up to 40-45°C and keep it warm for 3 hours, and the reaction is complete by sampling and spotting. (TLC detection: silica gel GF254 thin-layer plate; developing agent: petroleum ether / ethyl acetate=1:2) the above reaction mixture was cooled to 25~35° C., and ethyl acetate (EA) (EA) ( 10 times, v / m), stirring for 30 minutes. Filter, and rinse the filter cake with ethyl acetate 2 to 3 times. The filtrate was transferred to a 500L reactor, and 10 times the volume of 10% sodium chloride solution was added, stirred for 5 minutes, and a green solid was precipitated, left to stand for 5 to 10 minutes, and the lower layer of water phase was released, and 10 times the volume of 10% chlorine was added. Sodium chloride solution, stirring for 5 minutes, standing ...
Embodiment 2
[0093] Embodiment 2: compound shown in preparation formula 2
[0094] Add 8kg of the compound shown in Formula 1, 84kg of acetic acid, and 80L of tetrahydrofuran into a 500L reactor, start stirring; add 11.2kg of iron powder, heat up to 45-50°C and keep it warm for 1.5 hours, and the reaction is complete by sampling and spotting. (TLC detection: silica gel GF254 thin-layer plate; developing agent: petroleum ether / ethyl acetate=1:2) the above reaction mixture was cooled to 25~35° C., and ethyl acetate (EA) (EA) ( 10 times, v / m), stirring for 30 minutes. Filter, and rinse the filter cake with ethyl acetate 2 to 3 times. The filtrate was transferred to a 500L reactor, and 10 times the volume of 10% sodium chloride solution was added, stirred for 5 minutes, and a green solid was precipitated, left to stand for 5 to 10 minutes, and the lower layer of water phase was released, and 10 times the volume of 10% chlorine was added. Sodium chloride solution, stirring for 5 minutes, stan...
Embodiment 3
[0095] Embodiment 3: compound shown in preparation formula 2
[0096] Add 8kg of the compound shown in Formula 1, 80kg of acetic acid, and 78L of tetrahydrofuran into a 500L reactor, start stirring; add 10kg of iron powder, heat up to 50-55°C and keep it warm for 2 hours, and the reaction is complete by sampling and spotting. (TLC detection: silica gel GF254 thin-layer plate; developing agent: petroleum ether / ethyl acetate=1:2) the above reaction mixture was cooled to 25~35° C., and ethyl acetate (EA) (EA) ( 10 times, v / m), stirring for 30 minutes. Filter, and rinse the filter cake with ethyl acetate 2 to 3 times. The filtrate was transferred to a 500L reactor, and 10 times the volume of 10% sodium chloride solution was added, stirred for 5 minutes, and a green solid was precipitated, left to stand for 5 to 10 minutes, and the lower layer of water phase was released, and 10 times the volume of 10% chlorine was added. Sodium chloride solution, stirring for 5 minutes, standing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com