A virtual screening method for human transthyretin interferents
A virtual screening technology for thyroxine protein, which is applied in the fields of instruments, calculations, electrical digital data processing, etc., can solve problems such as unsystematic screening strategies and methods, single prediction models, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
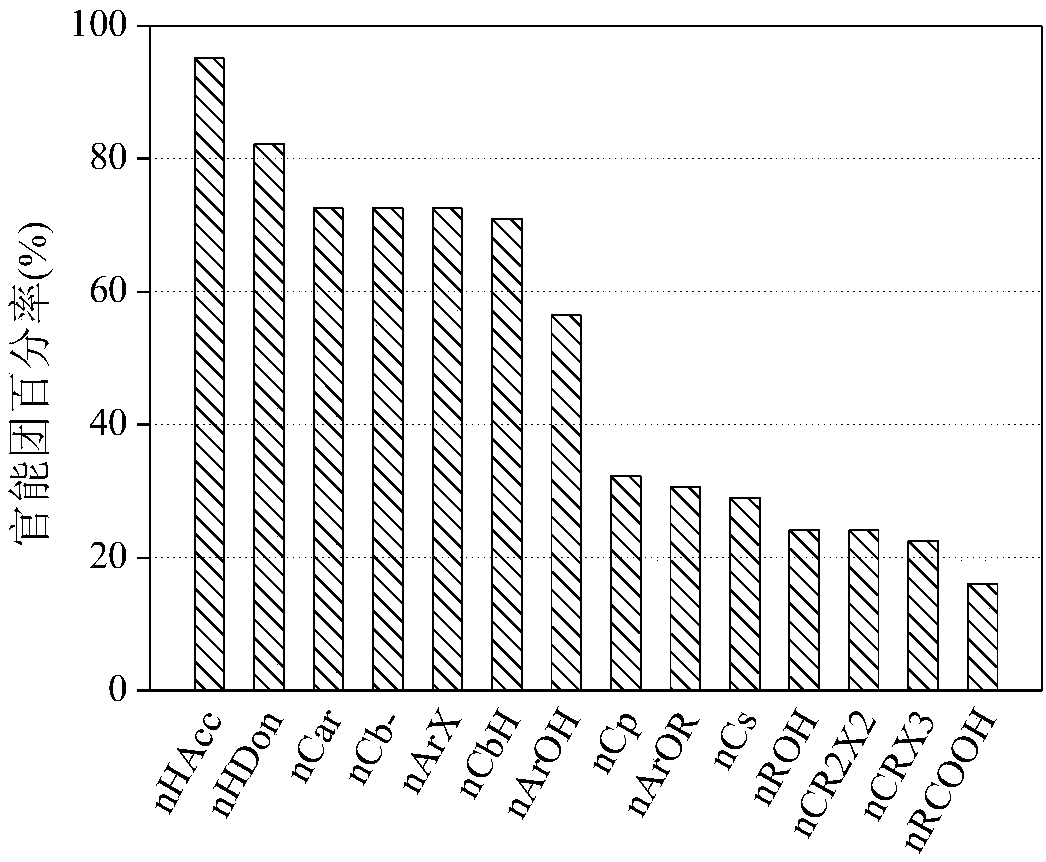
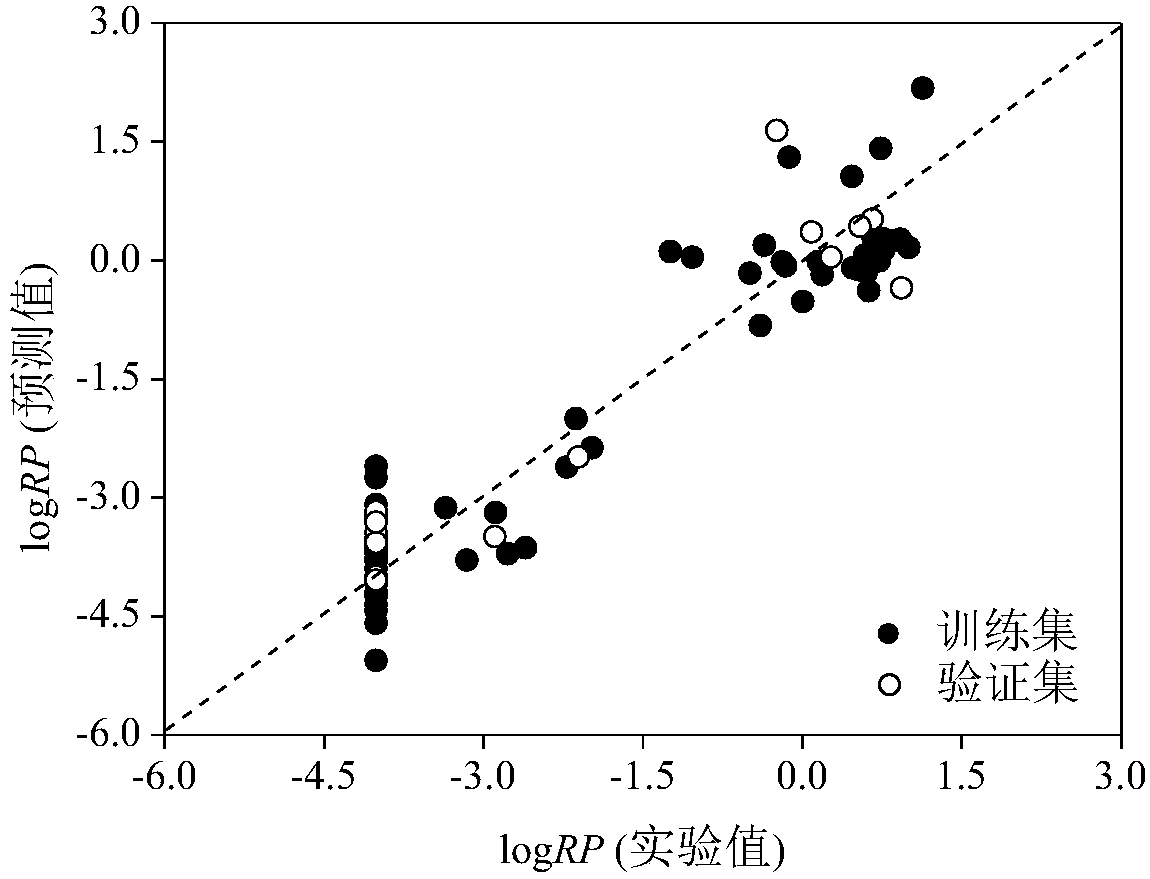
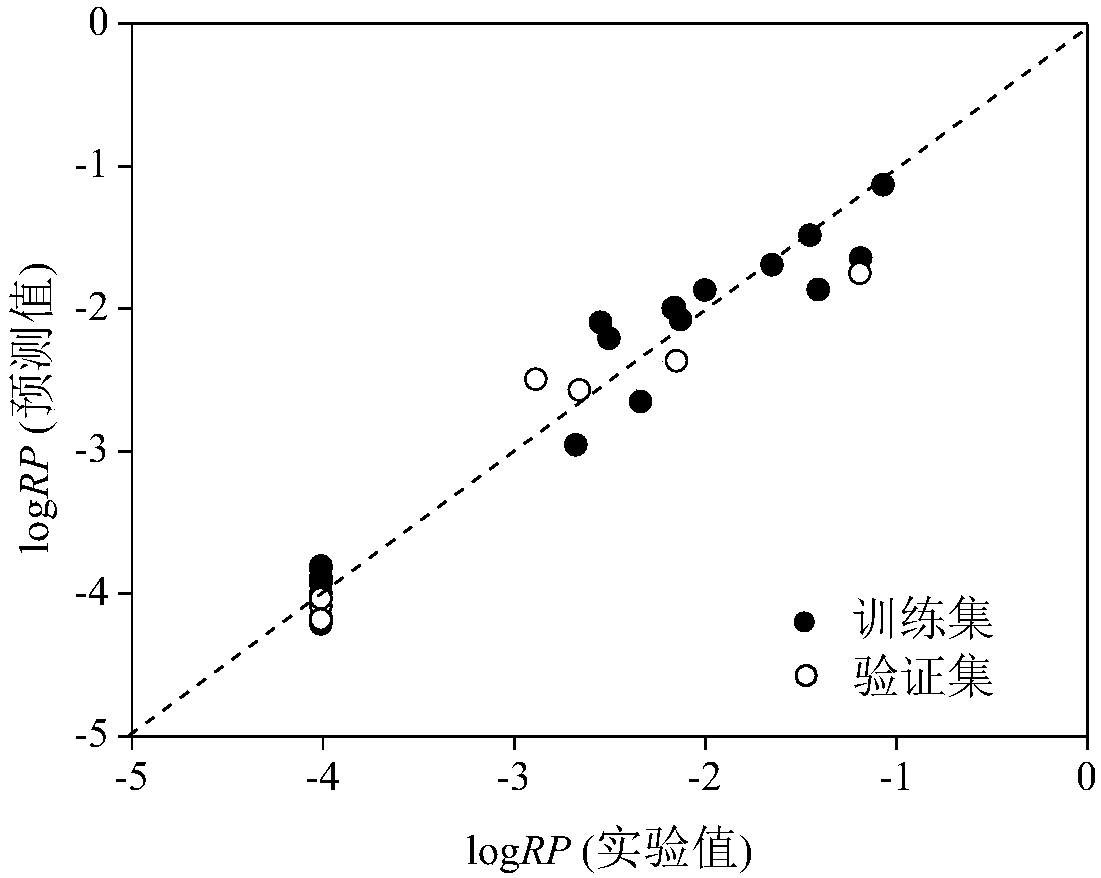
Image
Examples
Embodiment 1
[0089] 2,4,6-Tribromoaniline has no transthyretin interference activity (logRP=-4.011). The steps of utilizing the present invention to predict its interference activity are as follows:
[0090] Molecular descriptor calculated from Dragon, n of 2,4,6-tribromoaniline N =1, it is a nitrogen-containing organic chemical, which is not in the mechanism domain of the method, and cannot enter the next step of calculation.
[0091] The interfering activity of 2,4,6-tribromoaniline on human transthyretin could not be predicted using the model.
Embodiment 2
[0093] Interference activity of 2'-hydroxy-2,3',4,4'-tetrabromodiphenyl ether on human transthyretin logRP=-1.871×10 -1 . The steps of utilizing the present invention to predict its interference activity are as follows:
[0094] Molecular descriptor calculated from Dragon, n of 2'-hydroxy-2,3',4,4'-tetrabromodiphenyl etherN = 0, it is an organic chemical that does not contain nitrogen, within the mechanism domain of the method, and can enter the next step of calculation;
[0095] its n Car =12>0, it is an aromatic organic chemical, and its n ArOH +n ArCOOH =1>0, it is a phenolic organic chemical, which belongs to chemical category I within the mechanism domain of the method, and can enter the next step of calculation;
[0096] According to the classification results of chemicals, model 1 was selected for activity prediction.
[0097] According to the molecular structure of 2'-hydroxyl-2,3',4,4'-tetrabromodiphenyl ether, its leverage value is 0.03, which is within the appl...
Embodiment 3
[0105] The interference activity of 3,3',4,4',5-pentachlorobiphenyl on human transthyretin logRP=-3.354. The steps of utilizing the present invention to predict its interference activity are as follows:
[0106] Molecular descriptors calculated from Dragon, n for 3,3',4,4',5-pentachlorobiphenyl N = 0, it is an organic chemical that does not contain nitrogen, within the mechanism domain of the method, and can enter the next step of calculation;
[0107] its n Car =12>0, it is aromatic organic chemicals;
[0108] no ArOH +n ArCOOH = 0, not phenolic or benzoic organic chemicals;
[0109] no ROH +n RCOOH +n SO2OH +n SOOH =0, aromatic organic chemicals without hydroxyl or carboxyl or sulfonic acid or sulfinic acid groups;
[0110] no ArX =5>0, it is a halogenated aromatic organic chemical, which belongs to chemical category III within the mechanism domain of the method, and can enter the next step of calculation;
[0111] According to the classification results of chemi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com