Tadalafil oral soluble film agent and preparation method
A technology of tadalafil and orally dissolving film, which is applied to medical preparations with non-active ingredients, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as individual differences and tablet disintegration, and achieve Easy to take, easy to take, good uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
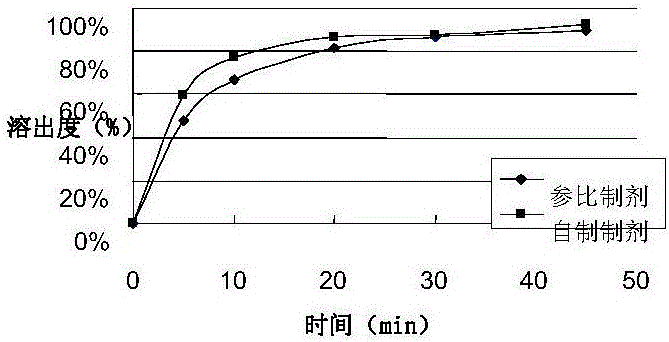
Embodiment 1
[0050] prescription:
[0051]
[0052] Preparation:
[0053] ① Handling of raw materials
[0054] The tadalafil bulk drug is mechanically pulverized first, and then jet pulverized, and the particle size (D90) is controlled below 5um.
[0055] ② Homogeneous emulsification process
[0056] Set the temperature of the water bath to 35°C, add 249.56g of purified water, 66g of ethanol and 0.4g of plasticizer into the homogenization pot, and stir evenly (3000rpm).
[0057] Slowly add 22g of flavoring agent, set the homogenization temperature to 25°C, and the homogenization speed to 3500rpm. After all the addition is complete, lower the lid and continue homogenization and stirring for 30 minutes. After the end, confirm whether it is fully dissolved. Otherwise, continue homogenization and use vacuum degassing for about 20min until the solution is clear.
[0058] Lift the lid, slowly add 20g of the processed raw material tadalafil, the homogenization speed is 3500rpm, the additio...
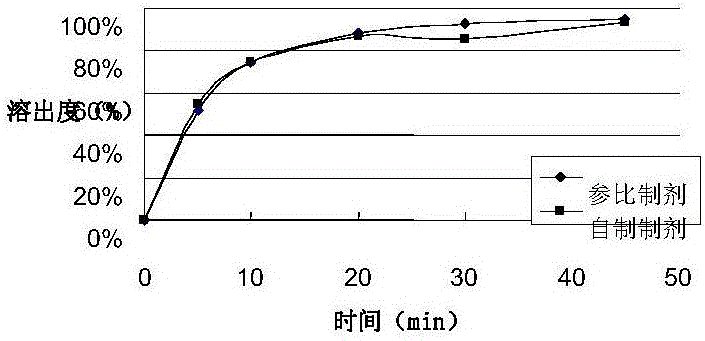
Embodiment 2
[0069] prescription:
[0070]
[0071]
[0072] Preparation:
[0073] According to the preparation process described in Example 1, the packaging specification is 20 mg / tablet.
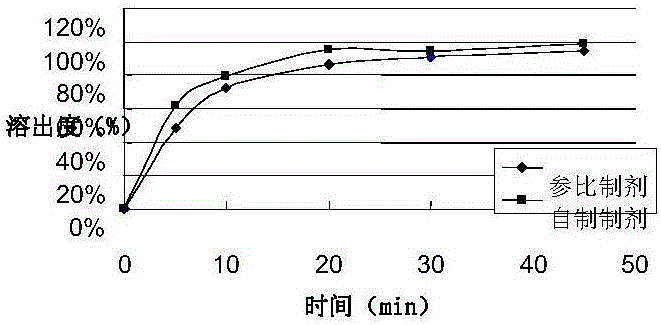
Embodiment 3
[0075] prescription:
[0076]
[0077] Preparation:
[0078] According to the preparation process described in Example 1, the packaging specification is 20 mg / tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com