Pharmaceutical composition and its preparation method
A composition and drug technology, applied in the field of pharmaceutical composition and its preparation method, can solve problems such as complicated manufacturing process, poor coating rate, drug leakage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of amphiphilic chitosan derivatives modified by hydrophobic hexanoyl group and hydrophilic carboxylic acid
[0041] First, 5 grams (g) of chitosan (Mw = 215,000 g / mol, 80 to 90% deacetylation, purchased from Adrich-Sigma) was suspended in isopropanol (50 milliliters (mL)) at room temperature , and stirred for 30 minutes. The resulting suspension was slowly mixed with aqueous sodium hydroxide solution (12.5 mL) to obtain a mixed solution, and the amount of grafted hydrophilic functional groups could be controlled by adjusting the concentration of sodium hydroxide in the mixed solution. Here, the mixed solution contained 13.3M sodium hydroxide. Next, the mixed solution was reacted with chloroacetic acid to prepare water-soluble hydrophilic carboxymethyl-modified chitosan, and dried.
[0042] 2 g of dried hydrophilic carboxyformic acid-modified chitosan was dissolved in pure water (50 mL), and stirred for 24 hours. Next, the obtained solution w...
Embodiment 2
[0044] Embodiment 2 Utilizes amphiphilic chitosan derivatives to coat medicine
[0045] Take by weighing the amphiphilic chitosan powder prepared in Example 1 of 0.5 milligrams (mg), add 200 microliters (μ L) of 0.1% demethoxycurcumin (dissolved in methanol), and utilize a shaker to mix slightly Finally, add 540 μL of double distilled water, then add 60 μL of pH 10.5 double distilled water, finally add 200 μL of 0.1% cisplatin (cisplatin) (dissolved in water), and stir for 12 hours to prepare the coated double drug (containing demethoxycurcumin and cisplatin) nanoparticles.
Embodiment 3
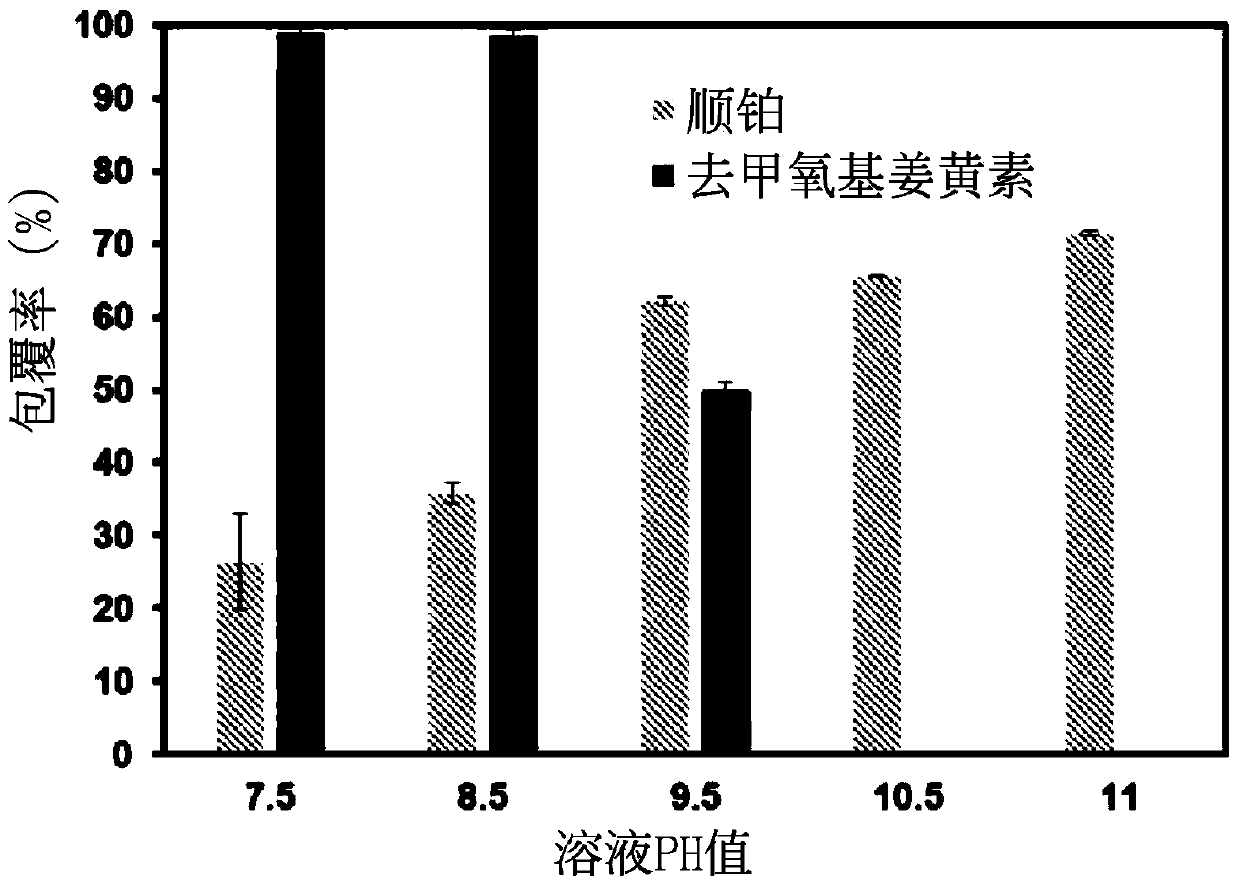
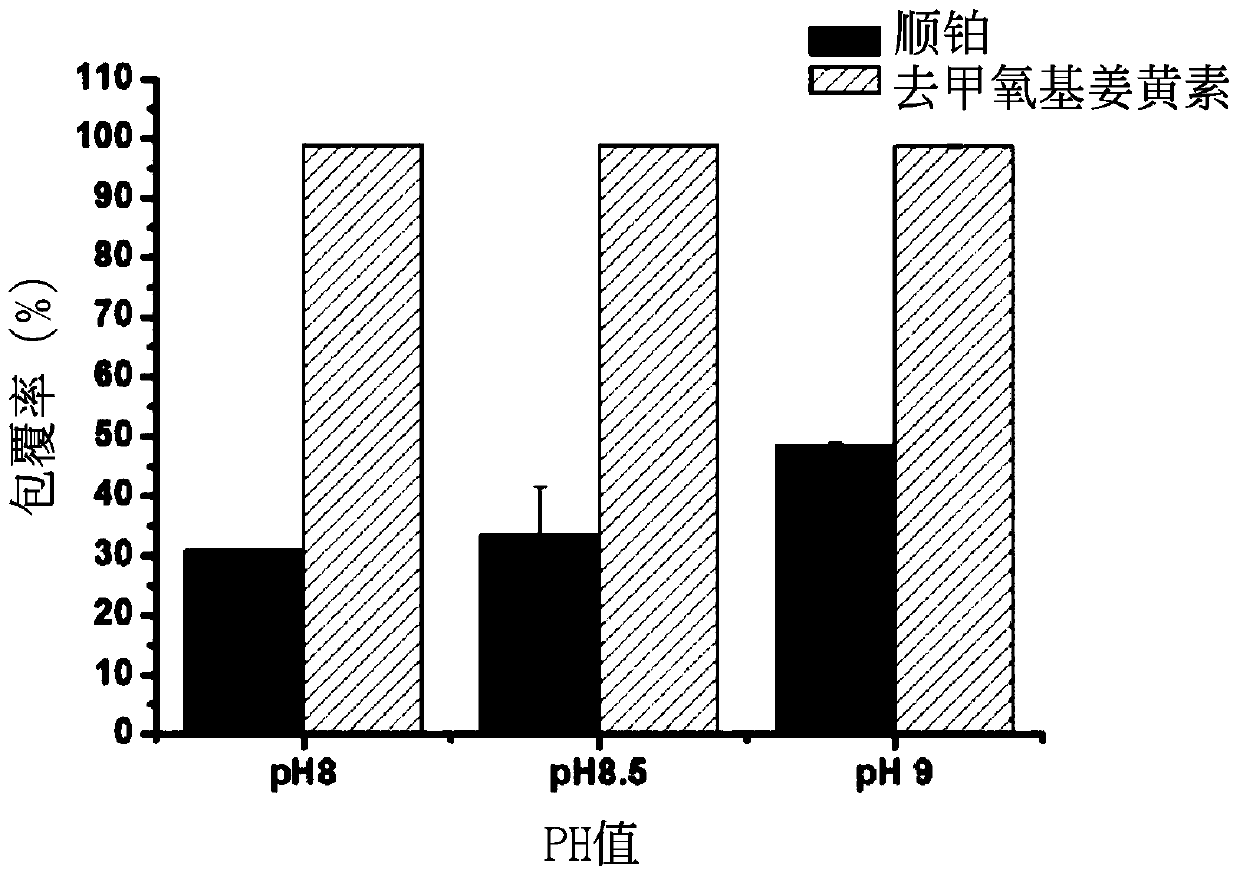
[0046] The coating rate test of the nanoparticle of embodiment 3 coating double drug
[0047] Put the synthesized double-drug coated nanoparticles into a concentrating centrifuge tube, and centrifuge at 4000 rpm for 30 minutes.
[0048] (1). Cisplatin coverage
[0049] Dilute the supernatant of the concentrated centrifuge tube by 20 times, mix it with 0.14% o-phenylenediamine in dimethylformamide solution at a ratio of 1:1, heat at 100°C for 30 minutes, and put it in a -20°C refrigerator for 10 minutes After cooling, the solution is finally detected by UV-visible light at the peak at a wavelength of 705nm. After conversion, the uncoated cisplatin content is obtained, and then the cisplatin coating rate is obtained.
[0050] (2). Demethoxycurcumin coating rate test
[0051] The nanoparticles on the filter membrane of the concentrated centrifuge tube were redissolved with twice distilled water, and the solution was mixed with methanol at a ratio of 1:1, and analyzed by HPLC. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com