Organic second-order nonlinear optical chromophore, synthetic method and application
A second-order nonlinear and chromophore technology, which is applied in the field of organic second-order nonlinear optical chromophores and their synthesis, can solve the problems of large intermolecular interactions, low polarization efficiency, and low solubility of polymer bases. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
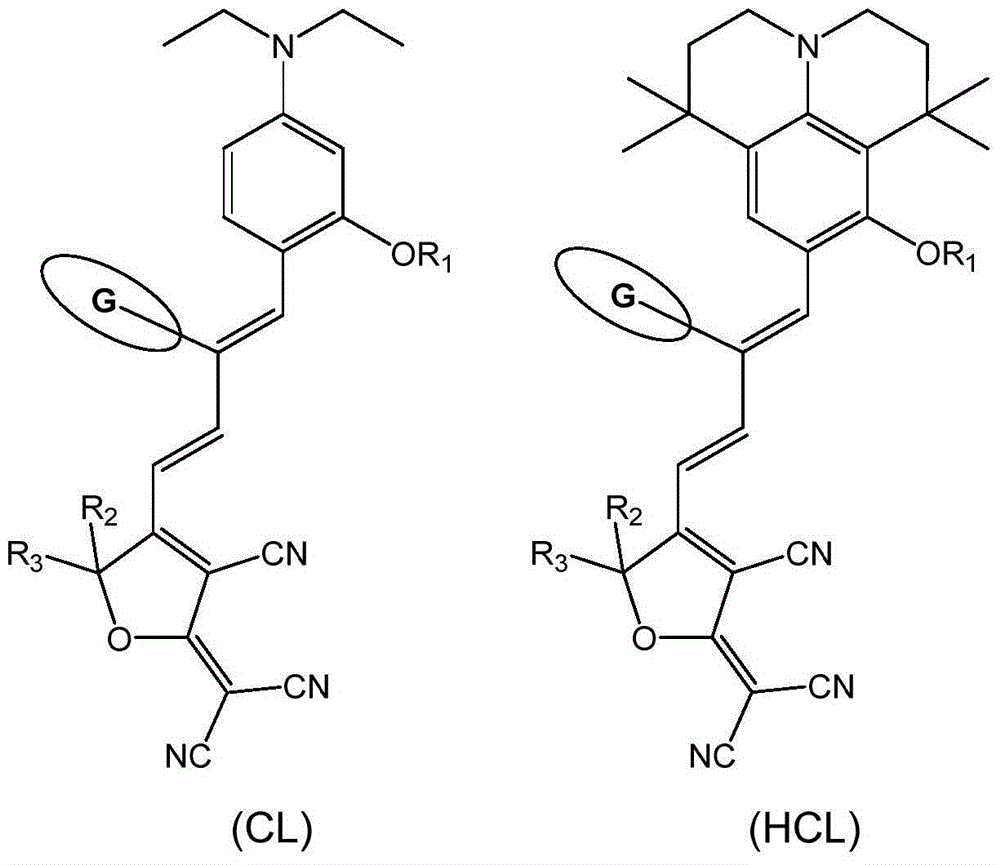
[0080] Synthesize the organic second-order nonlinear optical chromophore of D-π-A structure as shown below, it has formula (CL) structure:
[0081]
[0082] The synthetic route is as follows:
[0083]
[0084] The synthesis method is:
[0085] 1) Synthesis of compound 1 shown in the formula
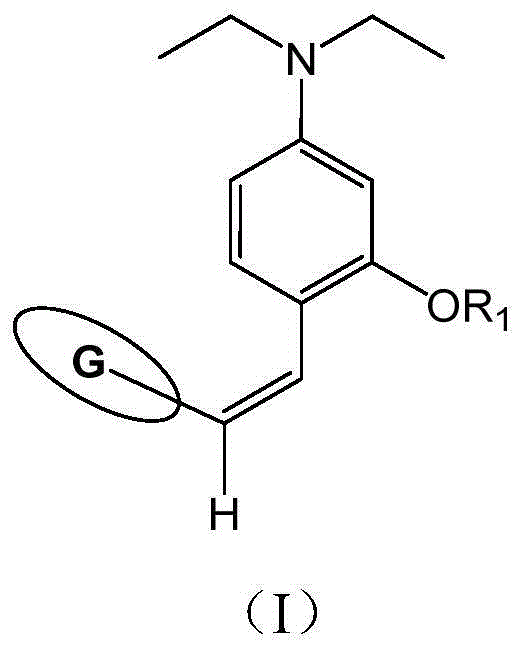
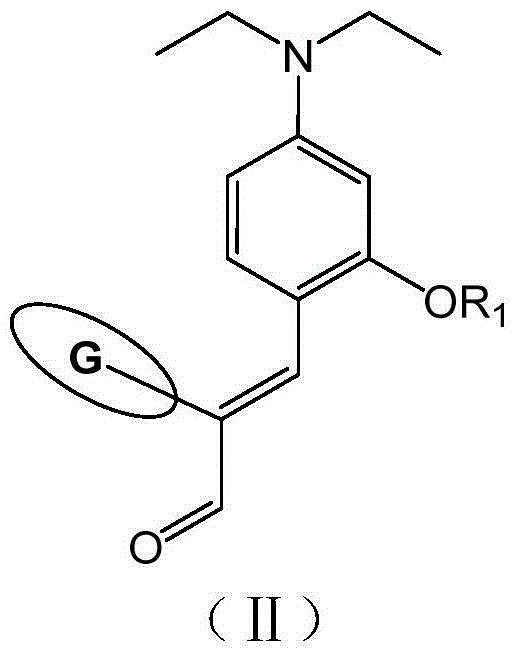
[0086] Add 3.86g (0.02mol) 4-(diethylamino)-2-hydroxybenzaldehyde, 3.60g (0.03mol) 1-chlorohexane and 60mL redistilled N,N-dimethylformaldehyde into a 100mL glass three-neck flask Amide, add 4.14g (0.03mol) of dried anhydrous potassium carbonate, reflux for 12 hours under nitrogen protection, observe the reaction progress by thin layer chromatography (TLC), cool and filter to remove potassium carbonate after the raw material point almost disappears, pour into 250mL saturated In salt water, extract with ethyl acetate, dry over night with anhydrous magnesium sulfate, filter, remove ethyl acetate by rotary evaporation, and separate the residue by column chromatography (using 200-300 ...
Embodiment 2
[0105] Synthesize an organic second-order nonlinear optical chromophore with the D-π-A structure shown below, formula (HCL1):
[0106]
[0107] The synthetic route is as follows:
[0108]
[0109] The synthesis method is:
[0110] 1) Synthesis of compound 1 shown in the formula
[0111] Add 2.73g (0.01mol) 8-hydroxy-1,1,7,7-tetramethyljulonidine-9-carbaldehyde and 2.33g (0.015mol) 1,6-dichlorohexyl to a 100mL glass three-neck flask Alkanes and 40mL redistilled N,N-dimethylformamide, added 2.07g (0.015mol) of dried anhydrous potassium carbonate, refluxed for 12 hours under the protection of nitrogen, and observed the reaction progress by thin layer chromatography (TLC). After almost disappearing, it was cooled and filtered to remove potassium carbonate, poured into 250mL saturated saline, extracted with ethyl acetate, dried over anhydrous magnesium sulfate and filtered, and rotary evaporated to remove ethyl acetate, and the residue was separated by column chromatography...
Embodiment 3
[0127] Synthesize an organic second-order nonlinear optical chromophore with the D-π-A structure shown below, formula (HCL2):
[0128]
[0129] The synthetic route is as follows:
[0130]
[0131] The synthesis method is:
[0132] 1) The synthesis of compound 1 shown in the formula is the same as in Example 2.
[0133] 2) Synthesis of compound 2 shown in the formula
[0134] Add 10.30g of triphenylphosphine hydrogen bromide (0.03mol), 4.98g (0.033mol) of 4-(dimethylamino)benzyl alcohol, and 200mL of chloroform dried with magnesium sulfate into a 250mL flask, and heat to reflux for 3-4 hours , spin dry. Add a small amount of chloroform to dissolve, pour into a large amount of anhydrous ether, and filter with suction to obtain a white solid with a yield of 95%.
[0135] 3) Synthesis of compound 3 shown in the formula
[0136] In a 100mL flask, add 3.92g (0.01mol) of compound 1 and 5.24g (0.011mol) of compound 2 (phosphine salt) in a molar ratio of 1:1.1 and dissolve t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com