Protein protectant
A technology of protein protectant and protectant, applied in the biological field, can solve the problem of long-term storage of Hsp90α protein that has not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
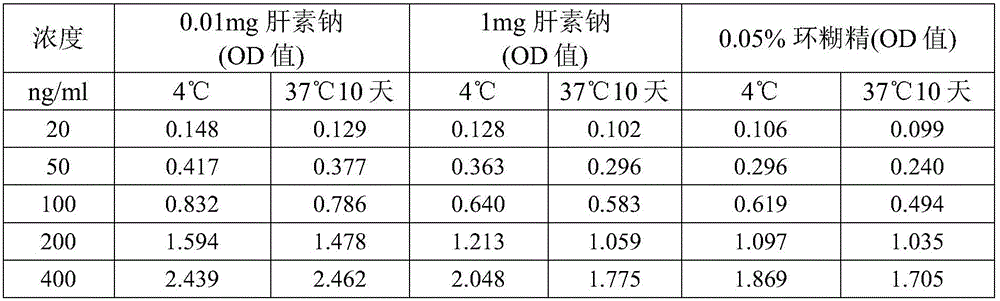
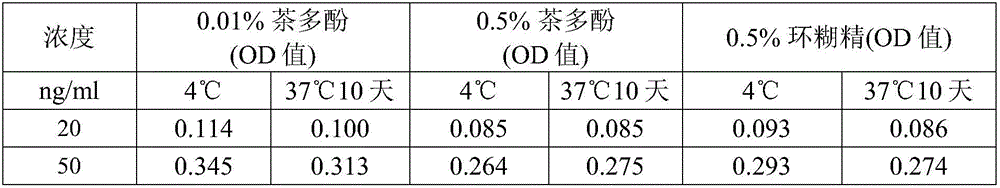
[0121] Example 1 Effect of adding a single component protective agent on the stability of Hsp90α protein
[0122] Objective: To screen new Hsp90α protein protectors, and to determine the concentration range and conditions for their use.
[0123] Reagents: Phosphate, Tween 20 and tea polyphenols were purchased from Sinopharm Chemical Reagent Co., Ltd., bovine serum albumin was purchased from Sigma Company, heparin sodium was purchased from Xinjing Biotechnology, and cyclodextrin was purchased from Beijing Aoboxing Biotechnology Co., Ltd. company. The Hsp90α finished kit was purchased from Yantai Proji Biotechnology Development Co., Ltd., batch number 201602002, 201604001, and the calibrator of the kit was Hsp90α freeze-dried product.
[0124] method:
[0125] 1. Prepare the basic solution, use purified water, which contains 0.318% phosphate (sodium dihydrogen phosphate), 0.5% bovine serum albumin and 0.2% Tween 20, adjust the pH to pH6.5, this solution is the basic of the lat...
Embodiment 2
[0151] Example 2 Effects of Combined Use of Multiple Protective Agents on the Stability of Hsp90α Protein
[0152] Objective: To develop a new combination formula of Hsp90α protein protector, and to determine its concentration range and conditions.
[0153] Reagents: Phosphate, Tween 20, MgCl 2 , tea polyphenols and K 2 SO 4 Purchased from Sinopharm Chemical Reagent Co., Ltd., bovine serum albumin and AMP were purchased from sigma, heparin sodium was purchased from Xinjing Biotechnology, PVP40 was purchased from Xinjing Biotechnology, and cyclodextrin was purchased from Beijing Aoboxing Biotechnology Co., Ltd. The Hsp90α finished kit was purchased from Yantai Proji Biotechnology Development Co., Ltd., batch number 201602002, 201604001, and the calibrator of the kit was Hsp90α freeze-dried product.
[0154] method:
[0155] The basic experimental method and steps are the same as those in Example 1, and in this example, a combination of multiple protective agents is used.
...
Embodiment 3
[0183] Example 3 Using the most effective protective agent combination formula to detect the performance index of the Hsp90α kit
[0184] Objective: To test the effect of the combination formulation of the most effective protective agent on the detection performance of Hsp90α protein and the kit through 12 days of accelerated experiment at 37°C.
[0185] Method: The detection was performed according to the operation steps in Example 1, wherein the accelerated Hsp90α freeze-dried product was used as the calibration product curve, and the quality control product was added at the same time to test the performance of the kit.
[0186] Results: The Hsp90α freeze-dried calibrator using the most effective combination formula is still stable after 12 days of acceleration, and the results of various performance indicators of the kit are shown in Table 7 below:
[0187] Table 7 The structure of each performance index of the kit
[0188]
[0189] Among them, the lowest detection limi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com