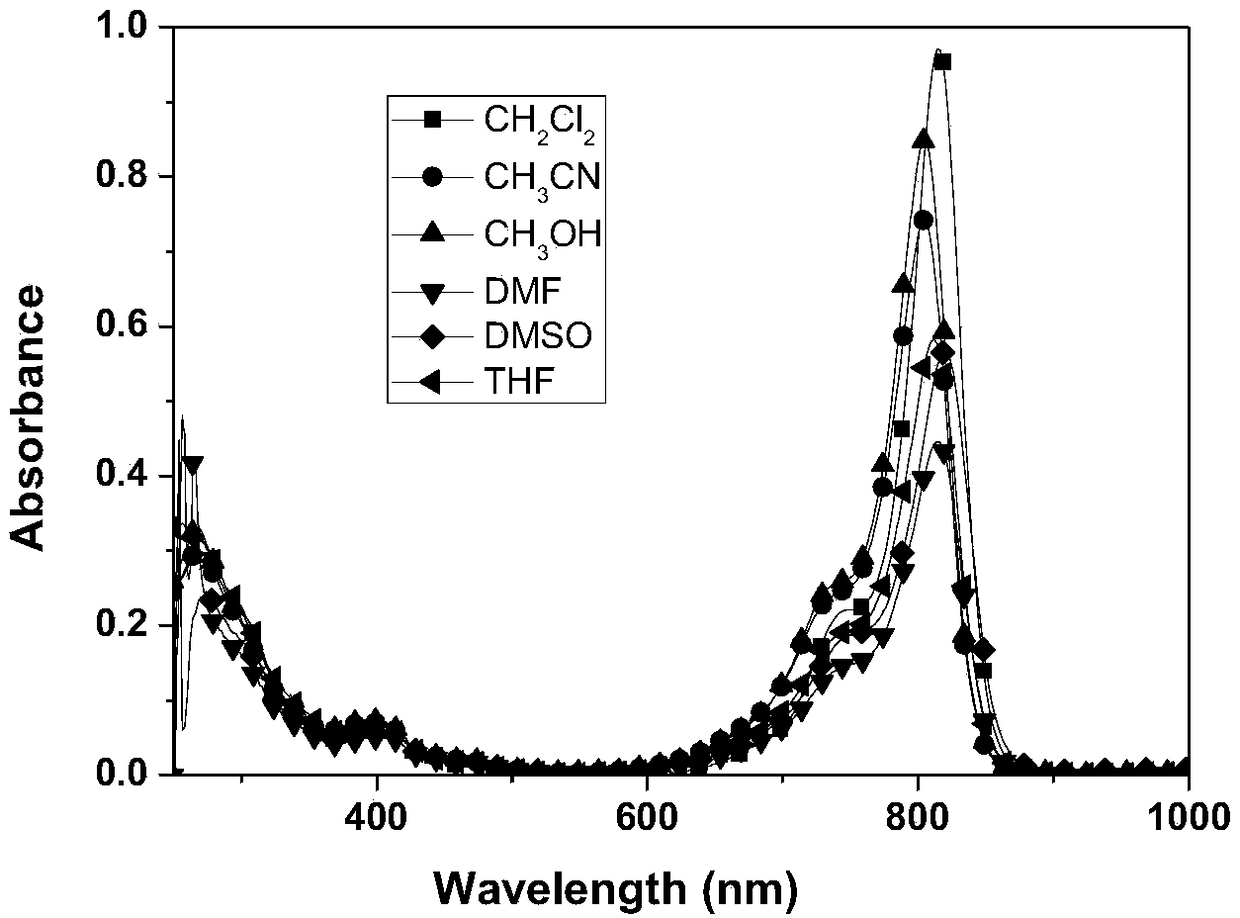
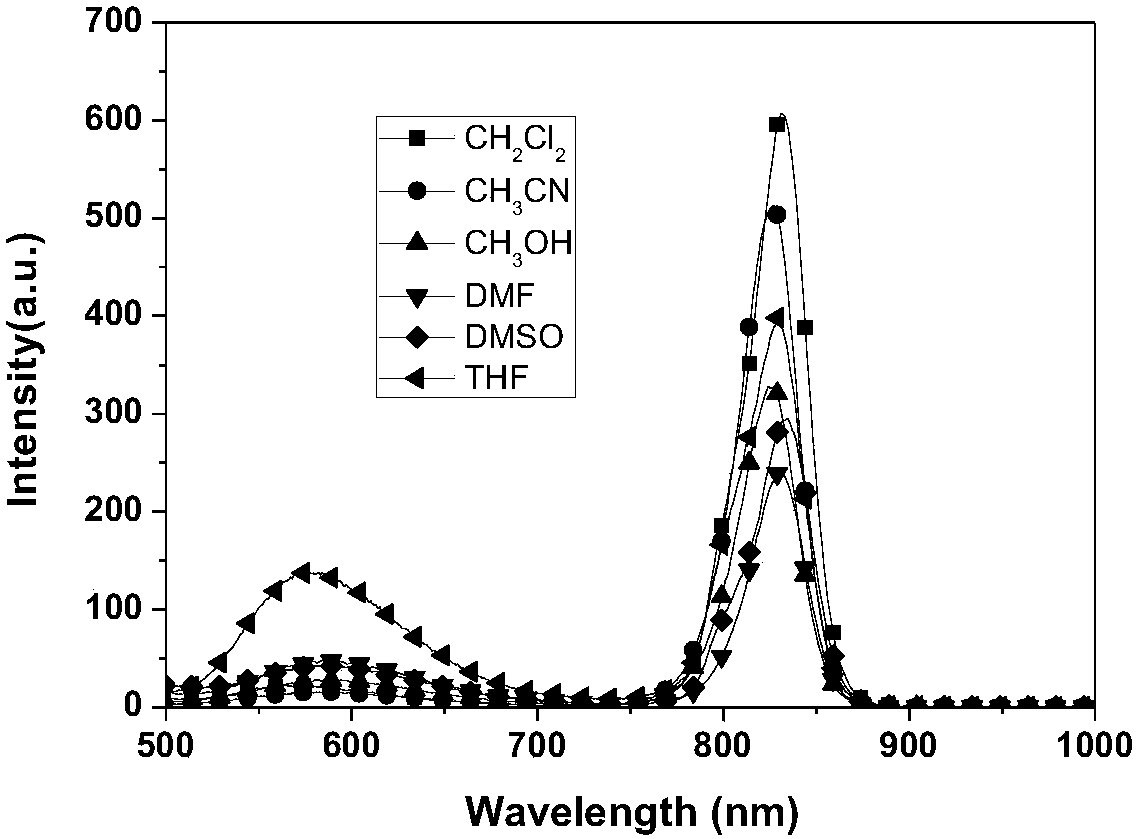
A kind of near-infrared metal iridium complex and its preparation method and application
A metal complex and near-infrared technology, which is applied in the fields of pharmaceutical formulations, indium organic compounds, chemical instruments and methods, etc., can solve the problems of unfavorable biological diagnosis and treatment application, single treatment effect, damage, etc., and achieve convenient and easy operation and reaction in the purification process. Effects in simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of 1,10-phenanthroline-5,6-dione:
[0036] In the three-necked flask, add a mixture of 1,10-phenanthroline (5g) and potassium bromide (25g) to mix uniformly, add 100mL of concentrated sulfuric acid and 50mL of concentrated nitric acid dropwise at 0°C, and slowly Raise the temperature to 80°C and react at 80°C for 2h. After the reaction is complete, pour into 2L crushed ice and neutralize to neutral with sodium hydroxide, extract the product from water with dichloromethane, and collect the dichloromethane layer. Spin dry to obtain a yellow solid. Recrystallized with methanol to obtain 1,10-o-phenanthroline-5,6-dione.
[0037] 1 H NMR (400MHz, DMSO-d6) δ9.03 (dd, J = 4.6, 1.8Hz, 2H), 8.43 (dd, J = 7.8, 1.8Hz, 2H), 7.71 (dd, J = 7.8, 4.7Hz, 2H).
Embodiment 2
[0039] Preparation of 4-(1-phenyl-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)phenol:
[0040] 1,10-phenanthroline-5,6-dione (3mmol,), ammonium acetate (36mmol,) and p-hydroxybenzaldehyde (3mmol,) were dissolved in 25mL of glacial acetic acid, and aniline (3.6mmol,) was added , reflux overnight. Cool to room temperature, pour into 50mL of water, adjust the pH to be neutral with 25% aqueous ammonia solution, filter out the solid with suction, extract the filtrate with dichloromethane, spin dry, finally combine with the solid, and recrystallize from methanol to obtain 4-( 1-phenyl-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)phenol.
[0041] 1 H NMR (400MHz, DMSO-d6) δ9.92(s, 1H), 9.12(dd, J=4.3, 1.8Hz, 1H), 9.05(dd, J=8.1, 1.8Hz, 1H), 8.98(dd, J=4.3, 1.6Hz, 1H), 7.91(dd, J=8.1, 4.3Hz, 1H), 7.81–7.72(m, 5H), 7.52(dd, J=8.5, 4.3Hz, 1H), 7.45(d ,J=8.7Hz,2H),7.37(dd,J=8.5,1.6Hz,1H),6.76(d,J=8.7Hz,2H).
Embodiment 3
[0043] Add 4-(1-phenyl-1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)phenol (1.6mmol) and NaH (1.6mmol) in the flask, and 25mL anhydrous DMF, stirred at room temperature for 1 h, added ethyl cyanine dye (0.8 mmol), stirred at room temperature for 24 h, extracted with DCM and water, freeze-dried, and column chromatography with a mixed solvent of dichloromethane and methanol , to obtain the imidazole ligand of the cyanine dye.
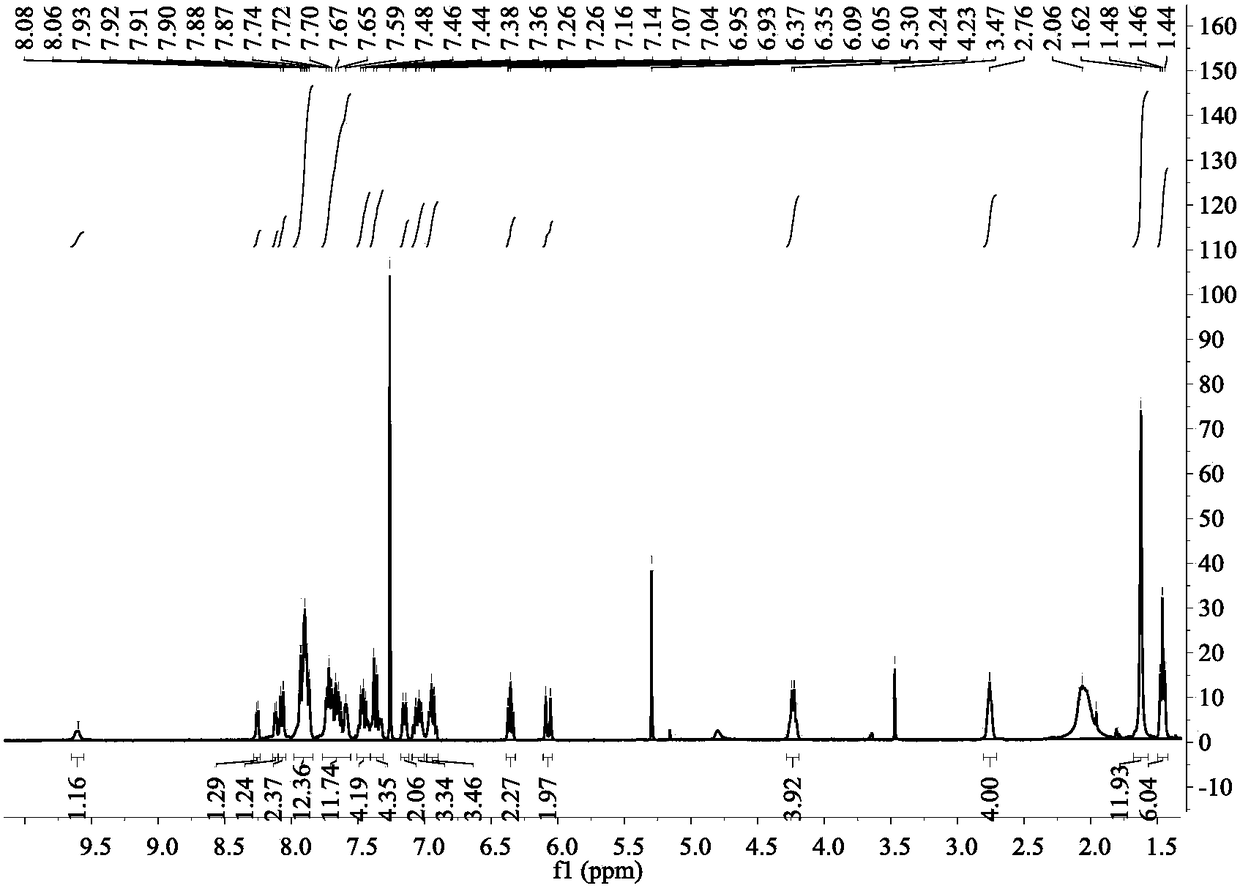
[0044] 1 H NMR (400MHz, CDCl 3)δ9.30(s,2H),9.09(s,1H),8.03(d,J=8.5Hz,2H),7.97–7.90(m,6H),7.86(dd,J=8.0,4.7Hz,1H ),7.76(d,J=8.5Hz,2H),7.68(t,J=7.4Hz,1H),7.61(s,4H),7.51–7.39(m,6H),7.32(d,J=2.9Hz ,2H),7.14(d,J=8.7Hz,2H),6.24(s,2H),4.36(d,J=7.2Hz,4H),2.83(s,4H),2.10(s,2H),1.65 (s,12H),1.49(t,J=7.2Hz,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com