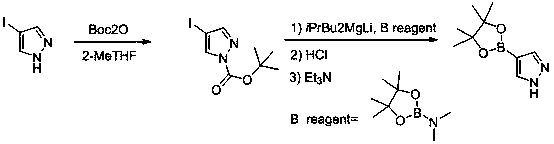
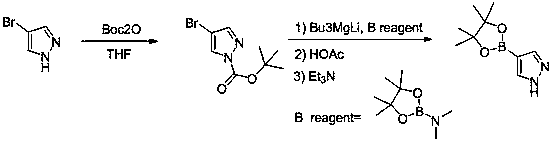
A method for synthesizing pyrazole-4-boronic acid pinacol ester
A technology for synthesizing pyrazoles and alcohol borates, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problem of increased reaction cycle, increased drying steps, and intermediates Stability and other issues, to achieve the effect of improved yield and product purity, simple operation of the process as a whole, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] In the reaction kettle, add 4-bromopyrazole (1.47kg, 10mol) and 6kg of tetrahydrofuran, stir to dissolve, heat to 30-40°C, slowly add di-tert-butyl dicarbonate (2.18kg, 10mol), dropwise The temperature rises slightly, and the temperature is controlled not to exceed 45°C. After the dropwise addition was completed, the mixture was stirred for 1-2 hours, and TLC confirmed that the reaction was complete. The reaction solution was distilled under reduced pressure until it stopped flowing, and 1.2kg of n-heptane was added to cool down to 0°C for beating, filtered, and dried to obtain 2.01kg of N-BOC-4-bromopyrazole, with a yield of 92%, HPLC: 98.3 %.
[0027] In the reaction kettle, add 4.5kg of tetrahydrofuran and 1-BOC-4-bromopyrazole (2.01kg, 9.2mol), stir for 0.5 hours, cool down to -20°C, and add 3.2mol of Bu3MgLi dropwise at -10°C to 0°C [Preparation Method: Add 1.0eq n-butylmagnesium chloride dropwise to 2.0 equivalent n-butyllithium at -10°C~0°C], TLC to...
Embodiment 2
[0029]
[0030] In the reaction kettle, add 4-iodopyrazole (1.94kg, 10mol) and 5kg of tetrahydrofuran, stir to dissolve, heat to 20-30°C, slowly add di-tert-butyl dicarbonate (2.18kg, 10mol), dropwise The temperature rises slightly, and the temperature is controlled not to exceed 35°C. After the dropwise addition was completed, the mixture was stirred for 1-2 hours, and TLC confirmed that the reaction was complete. The reaction solution was distilled under reduced pressure until it stopped flowing, and 1.2kg of n-heptane was added to cool down to 0°C for beating, filtered, and dried to obtain 2.65kg of N-BOC-4-iodopyrazole, with a yield of 90%, HPLC: 97.4 %.
[0031] In the reaction kettle, add 4.5kg of 2-methyltetrahydrofuran and 1-BOC-4-iodopyrazole (2.65kg, 9.0mol), stir for 0.5 hours, cool down to -20°C, keep -10°C to 0°C and add dropwise 2.95 mol Bu3MgLi [preparation method: add 1.0eq isopropylmagnesium chloride dropwise to 2.0 equivalents of n-butyllithium at -10°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com