Cefetamet pivoxil hydrochloride tablet composition
A technology of ceftazidime hydrochloride and mexil tablets, applied in the field of medicine, can solve problems such as patient harm and immediate allergic reaction, and achieve the effects of low polymer content, significant antibacterial activity, and strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
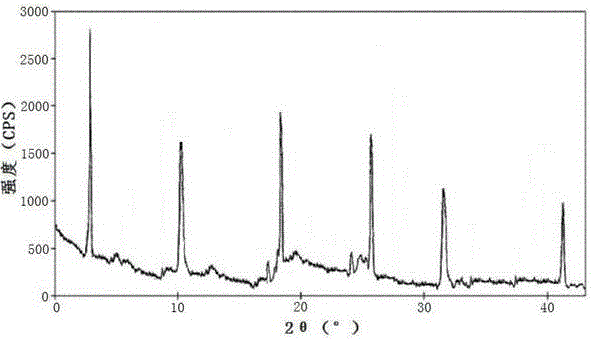
[0030] Embodiment 1: the preparation of ceftazidime pivoxil hydrochloride crystal
[0031] Get ceftamet pivoxil hydrochloride crude drug, add the mixed solution that volume is 8 times of ceftamet pivoxil hydrochloride weight diethyl ether and ethanol composition, wherein the volume ratio of diethyl ether and ethanol is 4:2.5, be heated to 30-35 ℃; Hydrochloric acid After the ceftazime pivoxil raw material is dissolved, add activated carbon for decolorization, and filter; the filtrate is heated and kept at a temperature of 35-40°C, and a mixed solvent of dimethylformamide and acetone whose volume is 6 times the weight of ceftamet pivoxil hydrochloride is added dropwise, The volume ratio of dimethylformamide and acetone is 2:1; after dropping, stir and cool down, the stirring and cooling is to cool down to 20-25°C at a speed of 1.5-3.5°C / min under stirring at a speed of 50-75rmp, and then Cool down to 5-10°C at a speed of 0.5-1°C / min under stirring at a speed of 30-45rmp, let st...
Embodiment 2
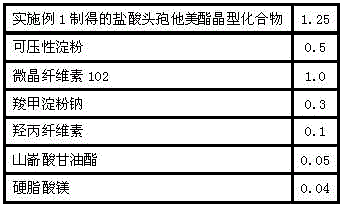
[0033] Embodiment 2: the preparation of ceftazidime pivoxil hydrochloride tablet, the steps are as follows:
[0034] Prescription: in parts by weight
[0035]
[0036] Preparation:
[0037] 1) Processing of raw and auxiliary materials: Use a vibrating sieving machine to pass compressible starch, microcrystalline cellulose 102, and hydroxypropyl cellulose through a 60-mesh sieve, carboxymethyl starch sodium through a 120-mesh sieve, and ceftazidime pivoxil hydrochloride through a 80-mesh sieve .
[0038] 2) Weighing: Weigh all raw and auxiliary materials according to the prescription;
[0039] 3) Mixing: Add the weighed raw and auxiliary materials into the mixer, set the motor operating frequency to 200r / min, and start the mixer to mix for 25 minutes;
[0040] 4) Choose a high-speed tablet press to press the tablet, adjust the pressure so that the tablet can be formed and the hardness is 4-9kgf, and the friability is not more than 1%;
[0041] 5) Packaging.
Embodiment 3
[0042] Embodiment 3: the preparation of ceftazidime pivoxil hydrochloride tablet, the steps are as follows:
[0043] Prescription: in parts by weight
[0044]
[0045] Preparation:
[0046]1) Processing of raw and auxiliary materials: Use a vibrating sieving machine to pass compressible starch, microcrystalline cellulose 102, and hydroxypropyl cellulose through a 60-mesh sieve, carboxymethyl starch sodium through a 120-mesh sieve, and ceftazidime pivoxil hydrochloride through a 80-mesh sieve .
[0047] 2) Weighing: Weigh all raw and auxiliary materials according to the prescription;
[0048] 3) Mixing: Add the weighed raw and auxiliary materials into the mixer, set the motor operating frequency to 200r / min, and start the mixer to mix for 25 minutes;
[0049] 4) Choose a high-speed tablet press to press the tablet, adjust the pressure so that the tablet can be formed and the hardness is 4-9kgf, and the friability is not more than 1%;
[0050] 5) Packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com