A kind of preparation method of adefovir dipivoxil hydroxymethyl impurity
A technology of adefovir dipivoxil and ester hydroxymethyl, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of wasting raw materials, and achieve the effects of reducing environmental protection costs, saving costs, and reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
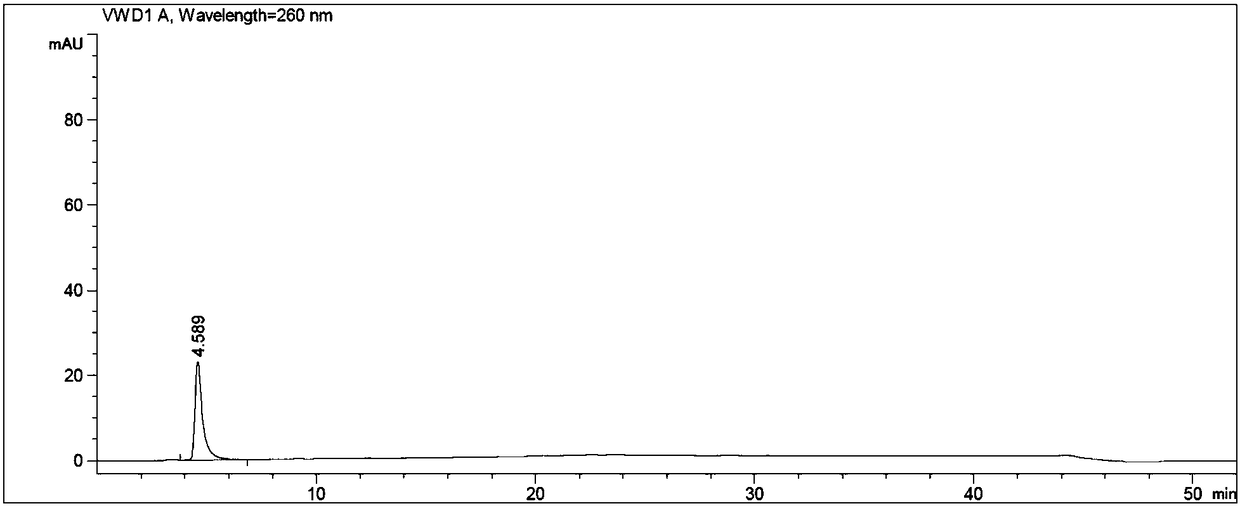
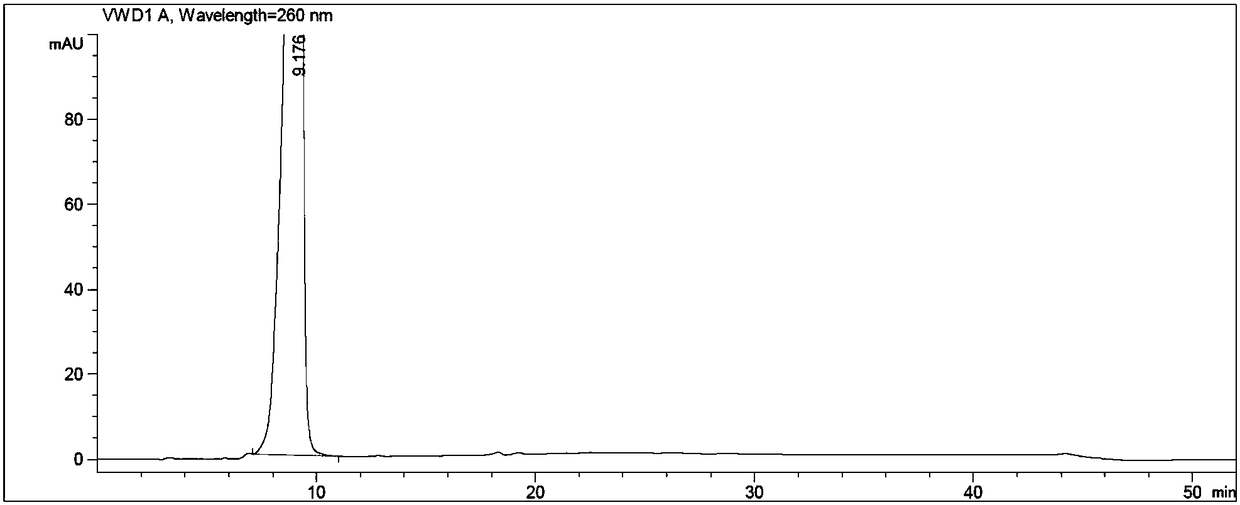
[0056] An adefovir dipivoxil hydroxymethyl impurity is prepared from the following raw materials through the following steps carried out in sequence according to the following weight ratio;
[0057]
[0058] Its chemical reaction formula is as follows:
[0059]
[0060] Put N-methylpyrrolidone, adefovir, triethylamine, and chloromethyl pivalate in turn into the reaction flask, stir, heat, control the temperature at 60±3°C, and react for 3 hours. After the reaction is complete, cool to room temperature. Add ethyl acetate, stir, and filter. The filtrate was washed with water, the organic layer was retained, the aqueous layer was separated, and the aqueous layer was extracted twice with ethyl acetate, and the organic layers were combined. The organic layer was washed twice with water, and the aqueous layer was discarded. The ethyl acetate layer was extracted twice with 2mol / L hydrochloric acid, the lower layer of hydrochloric acid layer (to prepare adefovir dipivoxil), the ...
Embodiment 1
[0064] Put 5.2g of N-methylpyrrolidone, 1.6g of adefovir A-4, 2.05g of triethylamine, and 4.4g of chloromethyl pivalate into a 250ml reaction bottle in sequence, stir, heat, and control the temperature at 60±3°C. React for 3h and cool to 30±5°C. Add 70ml of ethyl acetate, stir for 1h, and filter. The filtrate was transferred to a separatory funnel, added 20ml of drinking water to wash, the organic layer was retained, the water layer was separated, transferred to a separatory funnel, the water layer was extracted twice with ethyl acetate (20ml of ethyl acetate each time), and the organic layers were combined. The organic layer was washed twice with drinking water in a separating funnel (40ml of drinking water each time), and the water layer was discarded. The ethyl acetate organic layer was extracted twice with 2mol / L hydrochloric acid (the dosage of 2mol / L hydrochloric acid was 20ml each time), the lower hydrochloric acid layer was reserved (to prepare adefovir dipivoxil), th...
Embodiment 2
[0076] Put 104g of N-methylpyrrolidone, 32g of adefovir A-4, 41g of triethylamine, and 88g of chloromethyl pivalate into a 1L reaction bottle in sequence, stir, heat, control the temperature at 60±3°C, react for 3 hours, and cool to 30±5°C. Add 700ml of ethyl acetate, stir for 1h, and filter. The filtrate was transferred to a separatory funnel, added 400ml of drinking water to wash, the organic layer was retained, the water layer was separated, transferred to a separatory funnel, the water layer was extracted twice with ethyl acetate (400ml of each ethyl acetate consumption), and the organic layers were combined. The organic layer was washed twice with drinking water in a separating funnel (400ml of drinking water each time), and the water layer was discarded. The ethyl acetate organic layer was extracted twice with 2mol / L hydrochloric acid (the amount of 2mol / L hydrochloric acid was 400ml each time), the lower hydrochloric acid layer was reserved (to prepare adefovir dipivox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com