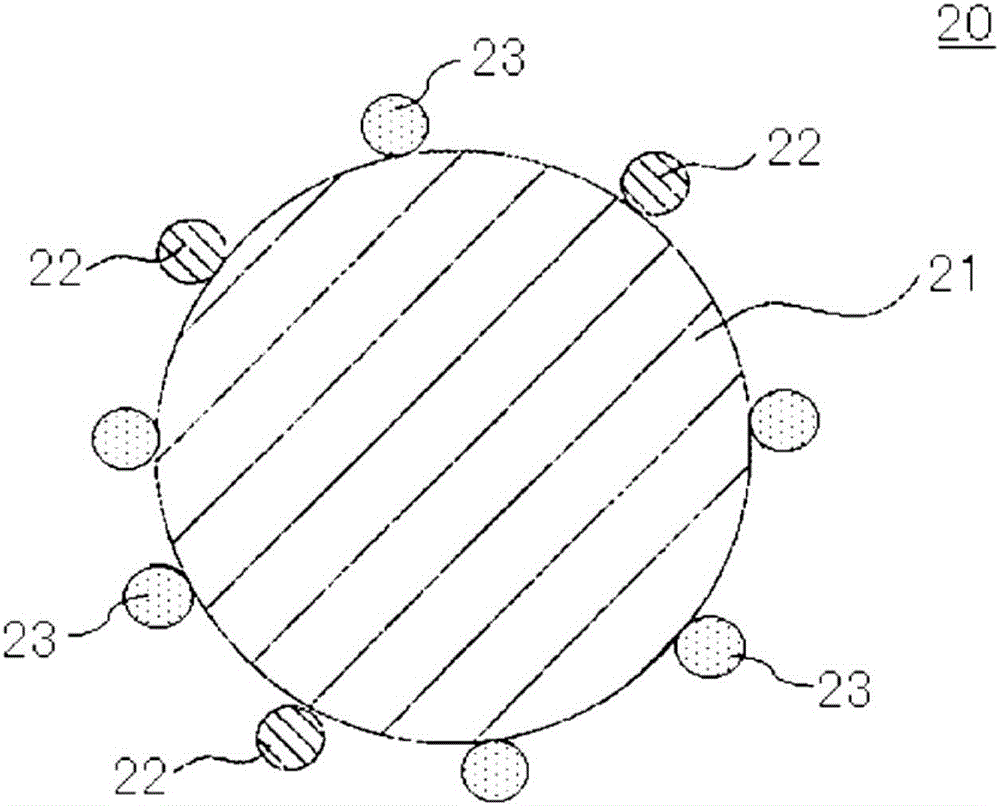
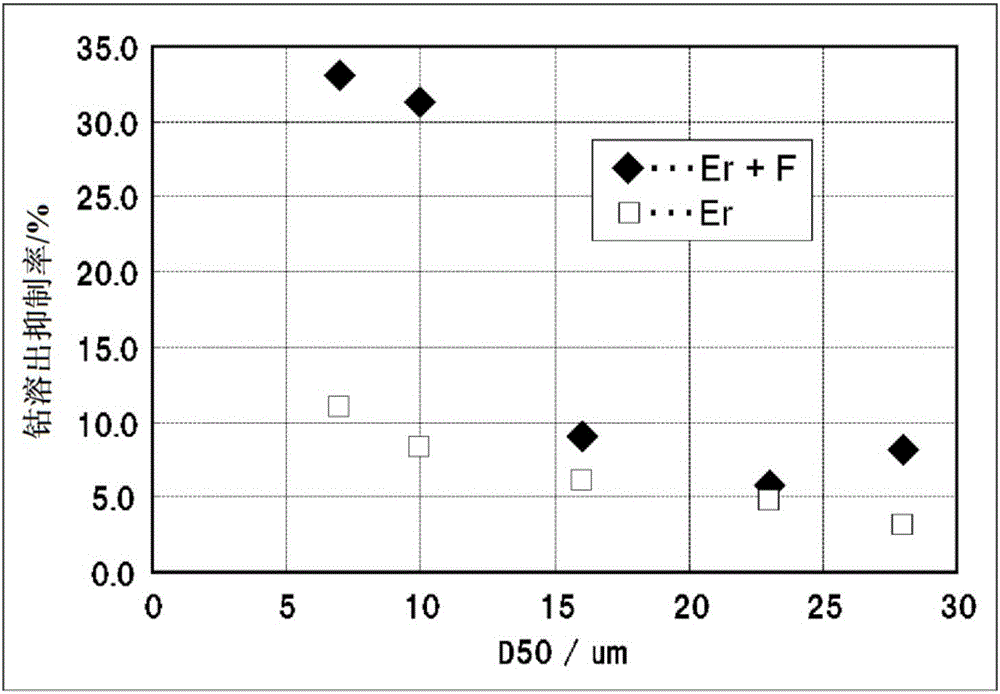
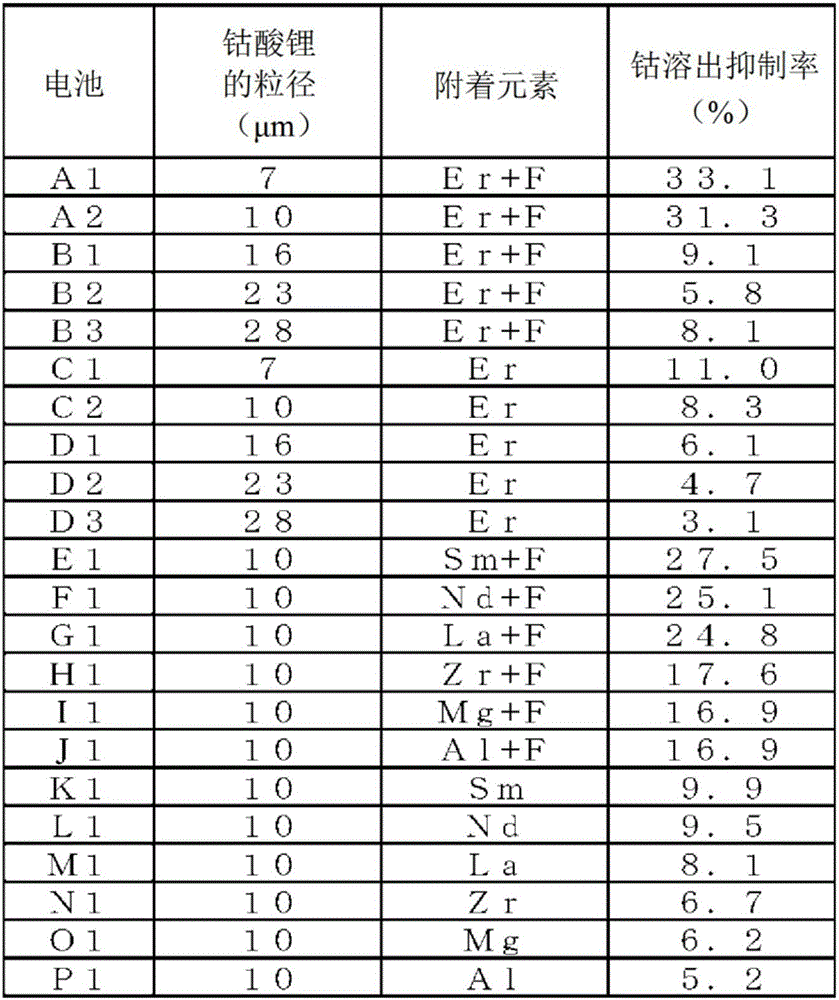
Positive-electrode active material for nonaqueous-electrolyte secondary battery and positive electrode for nonaqueous-electrolyte secondary battery
A technology of positive electrode active material and non-aqueous electrolyte, applied in the field of positive electrode active material for non-aqueous electrolyte secondary battery and positive electrode for non-aqueous electrolyte secondary battery, can solve the problems of reduced discharge capacity, easy decomposition of electrolyte, etc. Dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] [Preparation of non-aqueous electrolyte]
[0057] Lithium hexafluorophosphate (LiPF 6 ) was dissolved so as to have a concentration of 1.0 mol / liter to prepare a nonaqueous electrolyte (nonaqueous electrolyte solution).
[0058] [Production of battery]
[0059] Install lead terminals on the above-mentioned positive and negative electrodes respectively, arrange a separator between these two poles, and after winding into a spiral shape, pull out the winding core to make a spiral electrode body, and then flatten the electrode body to obtain a flat shape the electrode body. Next, this flat electrode body and the above-mentioned non-aqueous electrolytic solution were inserted into a case made of an aluminum laminate, and sealed to fabricate a battery A1. The design capacity (discharge capacity when charged to 4.40 V and discharged to 2.75 V) of the battery A1 is 750 mAh.
[0060] (Experiment 2)
[0061] Battery A2 was fabricated in the same manner as in Experiment 1 abo...
experiment example 11
[0079] Instead of erbium nitrate pentahydrate, use samarium nitrate hexahydrate (Sm(NO 3 ) 3 ·6H 2 (0) 1.14 g, and the battery E1 was produced in the same manner as in Experiment 2 above. The attached amounts of samarium and fluorine were 0.085% by mass and 0.029% by mass, respectively, and the molar ratio of samarium to fluorine was 1:3.
experiment example 12
[0081] Instead of erbium nitrate pentahydrate, use neodymium nitrate hexahydrate (Nd(NO 3 ) 3 ·6H 2 (0) 1.12 g, and the battery F1 was fabricated in the same manner as in Experiment 2 above. The adhering amounts of neodymium and fluorine were 0.074% by mass and 0.029% by mass, respectively, and the molar ratio of neodymium to fluorine was 1:3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com