Preparation method and application of BACE1 shearing high-titer antibodies
A cutting type and antibody technology, applied in the field of DNA recombination, can solve problems such as research difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
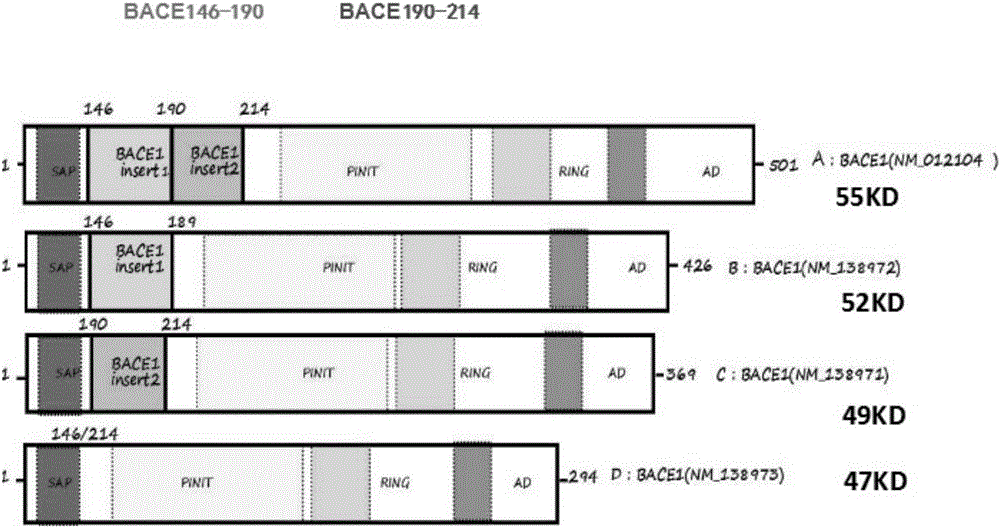
[0042] Example 1: Anti-GST-BACE1 190-214aa and GST-BACE1 146-189aa In preparation of spliced sera
[0043] 1. PCR-PMD18-T / BACE1 190-214aa and BACE1 146-189a Construction of recombinant plasmids
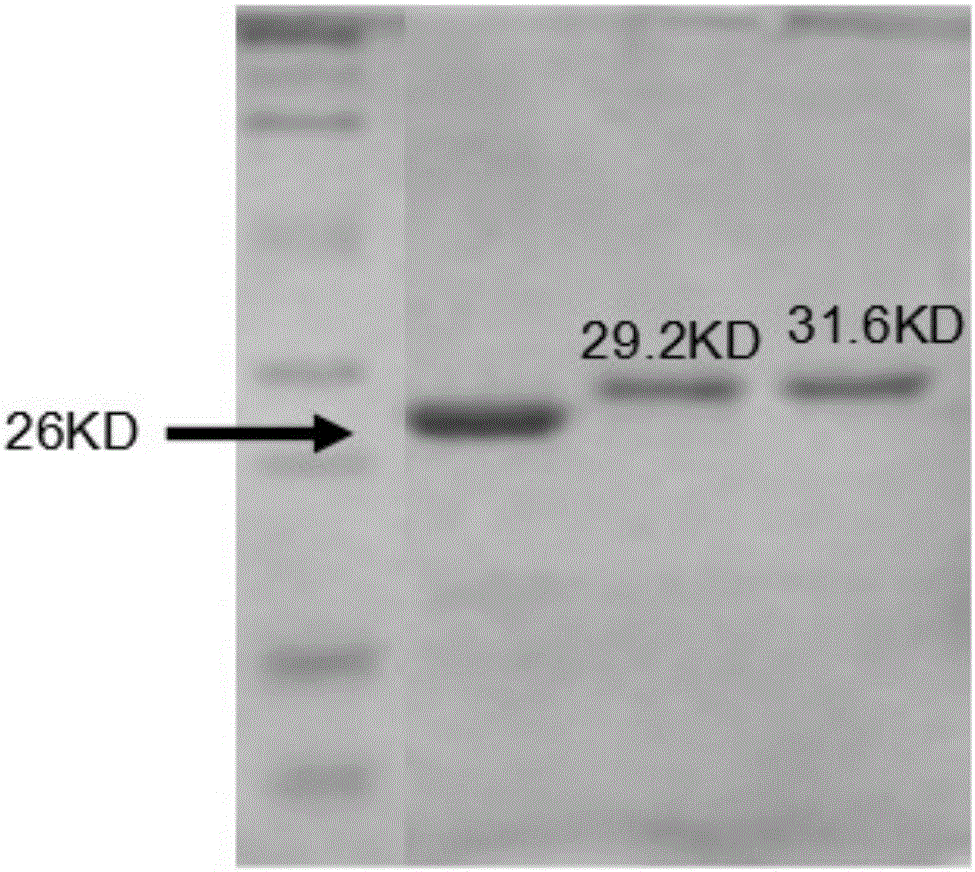
[0044] The full length of the BACE1 gene was obtained from Genebank, and the sequence number of the gene is NM 001207048. Using pCDNA3.1-Flag-BACE1 as template, 190-214aa upstream primer is GGA TCC ATG CCT GAC GAC TCC CTG GAG CCT TTCTTT GAC TCT CTG GTAAAG (including Bamh1 restriction site); downstream primer is 5'-CTC GAG CTA CTG CAG GGAGGA GAG GTT GGGAAC GTG GGT CTG CTT TAC CAG (with XhoI restriction site). 146-189aa The upstream primer is 5'-GGATCC ATG GTA AGC ATC CCC CAT GGC (containing the Bamh1 restriction site); the downstream primer is 5'-CTC GAGCTA CCT GGC AAT CTC AGC ATA (containing the XhoI restriction site). The DNA sequences corresponding to 75bp and 132bp of 25 and 44 amino acids on the BACE1 gene were successfully amplified by PCR.【 figure 2 】. The amplified f...
Embodiment 2
[0053] Example 2: GST-BACE1 190-214aa and GST-BACE1 146-189a Antibody analysis and application
[0054] 1. GST-BACE1 190-214aa and GST-BACE1 146-189a Determination of antibody titer
[0055] with GST-BACE1 190-214aa and GST-BACE1 146-189a New Zealand white rabbit serum before fusion protein immunization was used as a control, and the purified GST-BACE1 190-214aa and GST-BACE1 146-189a The antibody was first diluted 10 times and then doubled, and then the titer of the antibody was determined by indirect ELISA. The results showed that the anti-fusion protein GST-BACE1 was not detected in the rabbit serum before immunization 190-214aa and GST-BACE1 146-189a Antibody to GST-BACE1 190-214aa and GST-BACE1 146-189a The titer of the antibody is as high as 1:100000 or more【 Figure 6 】.
[0056] 2. GST-BACE1 190-214aa and GST-BACE1 146-189a Application of antibody to detection of BACE1 spliced protein in cells
[0057] Two high titer sera of rabbit anti-mouse GST-BACE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com