Agent for improving or preventing progression of chronic kidney disease
A chronic kidney disease and improver technology, applied in the direction of medical preparations containing active ingredients, pill delivery, organic active ingredients, etc., can solve the problems of no comparison of drug efficacy, no further development, and the effect cannot be said to be significant, etc., to achieve Easy to take, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
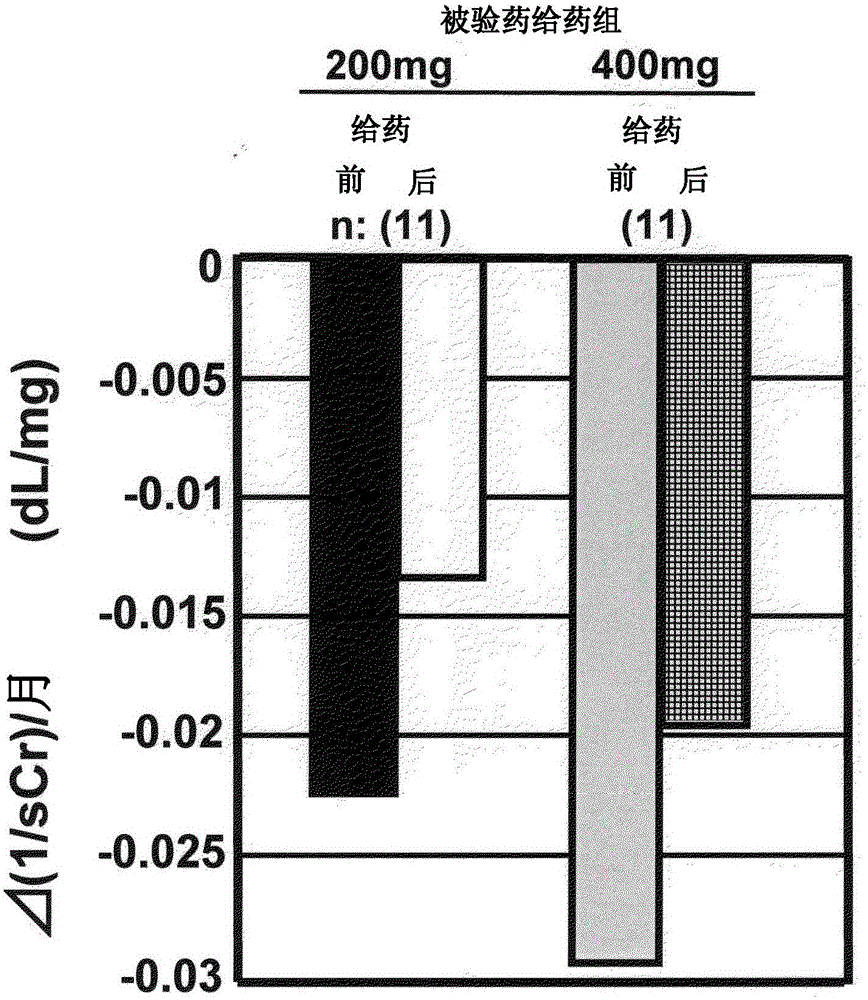
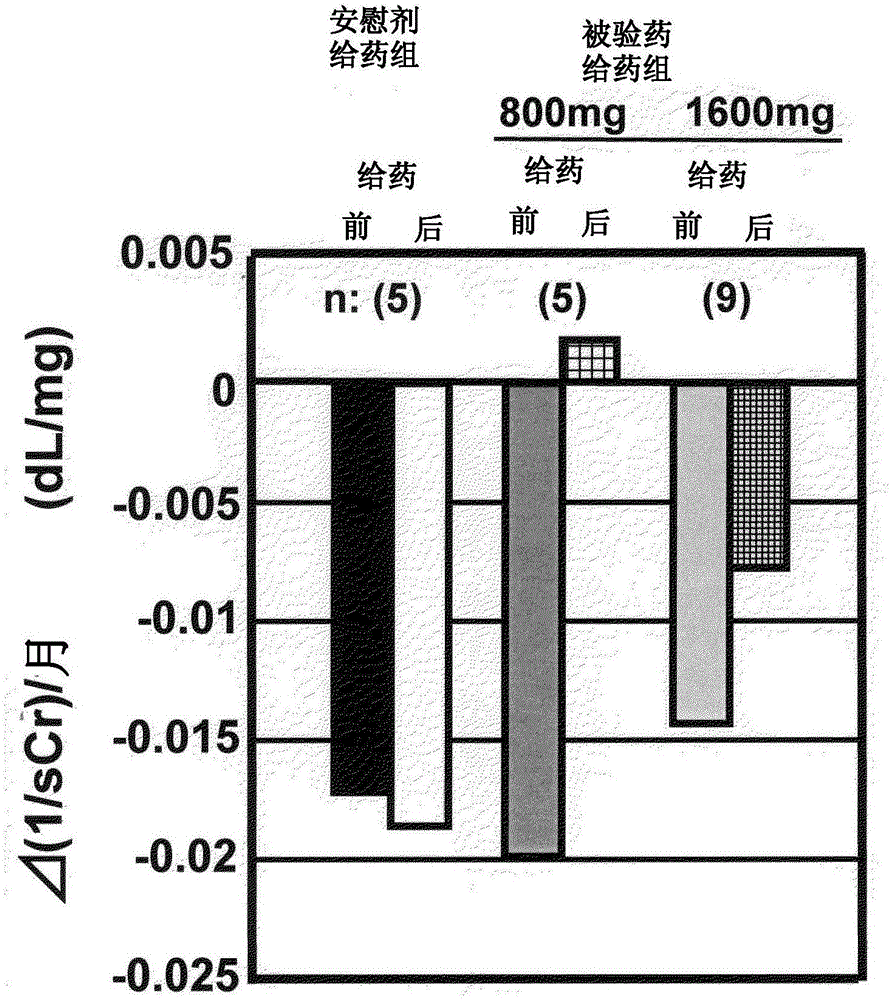
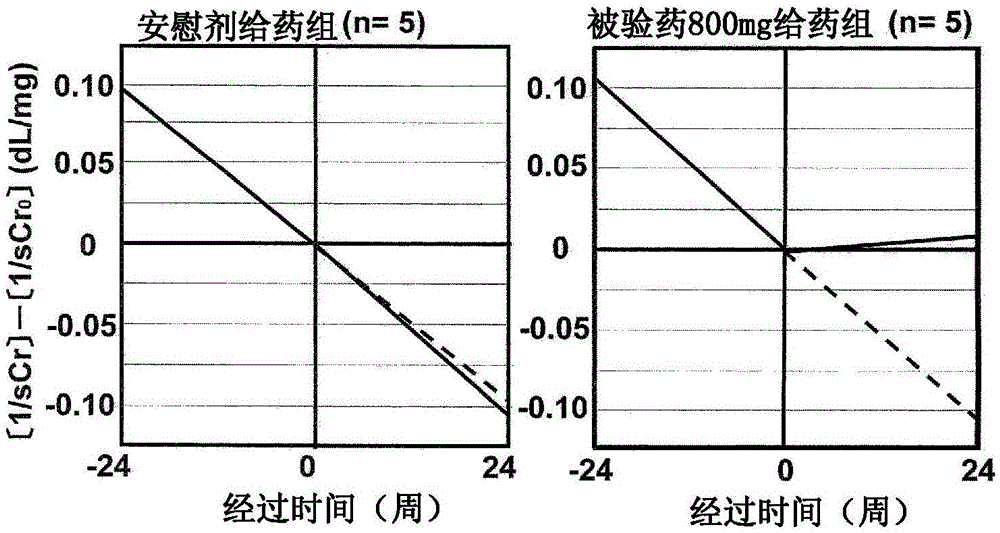
[0045] Next, the results of clinical trials using this drug (dosage form is a tablet) are shown. First, as a reference example, the results of a clinical test conducted before are illustrated, and next, as an example, the results of a clinical test conducted recently are illustrated to specifically explain the meaning of the present invention, but the present invention is not limited thereto.
[0046] Reference example Clinical trial results (1)
[0047] Clinical trials shown in Non-Patent Documents 1 and 2 will be described. Non-Patent Document 2 is a continuation report of the clinical trial of Non-Patent Document 1. The clinical trials involved in these two documents are referred to as "old clinical trials" below.
[0048] In the previous clinical trial, the test drug was orally administered once a day for 24 weeks to patients with diabetic CRF, and the efficacy and safety of this compound were evaluated. The test subjects were patients receiving conservative therapy for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com