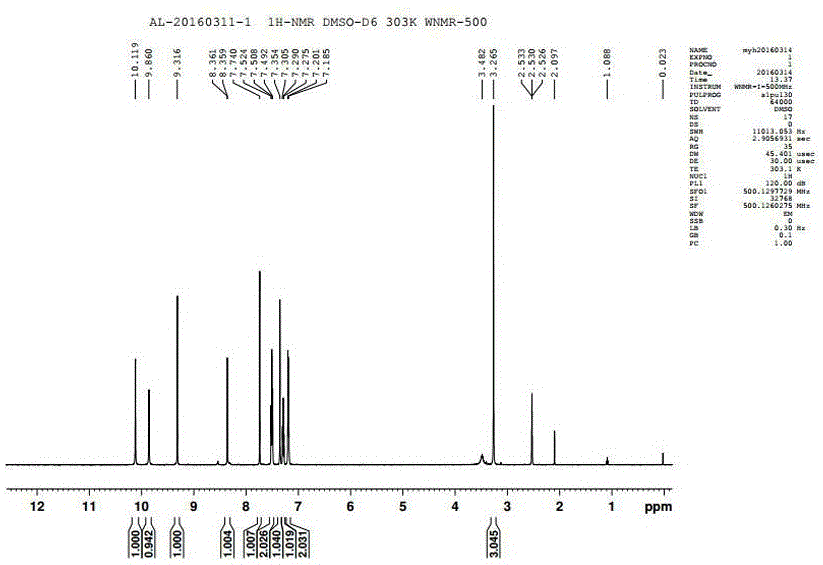
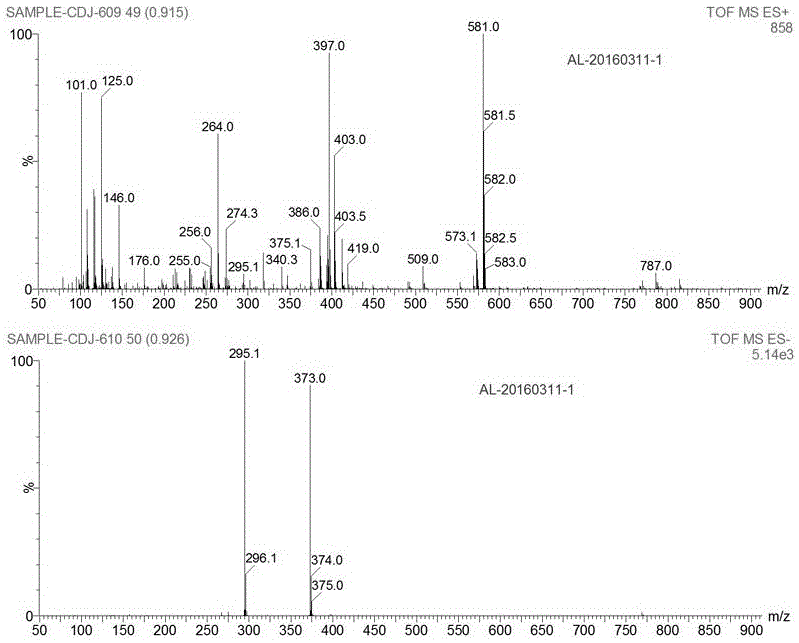
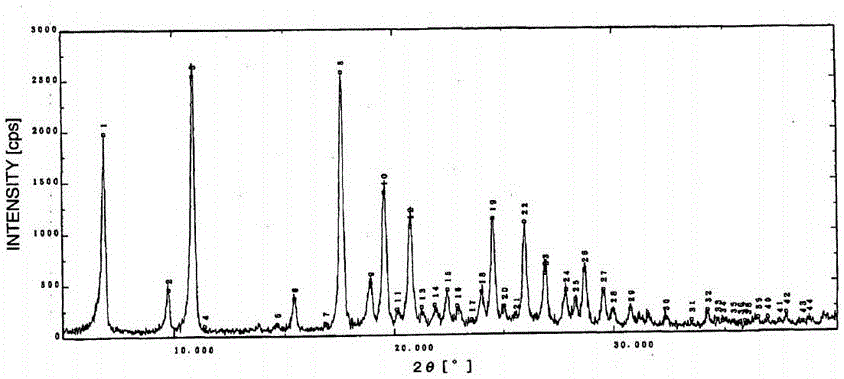
Preparation method of alpha crystal form of Iguratimod
A crystal form and crystallization technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of large ethanol consumption and unfavorable industrialization, and achieve the effect of reducing solvent consumption and facilitating industrial operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: raw material preparation mode
[0020] Add 200g of formamidomethyl-2-hydroxy-4-methanesulfonamido-5-phenoxyphenyl ketone and 300mL of N,N-dimethylformamide (DMF) into a 1L reaction flask. Add 200g of N,N-diformamide dimethyl acetal, 33.2g of glacial acetic acid, and 200mL of DMF successively under stirring. Stir at room temperature and react for 7 hours. After the reaction is over, transfer the material to a 10L glass beaker, stir, add 1L of dichloromethane and 1.5L of purified water to the reactor, and adjust the pH of the system to pH4 with 6N hydrochloric acid; after adjusting the pH, continue stirring to make the flocculent The solid precipitated out completely. After the precipitation is completed, filter until there is no continuous drop, discharge the material, and wash the filter cake successively with dichloromethane, purified water, and absolute ethanol for 3 to 5 minutes each, filter, and dry in vacuo to obtain the crude product of iguratimod....
Embodiment 2
[0023] Take 10g of the refined product of Alamode, put it into a 250mL eggplant-shaped flask; add 90ml of a 1:1 DMF / ethanol mixed solvent, reflux at 90°C for 30 minutes, let it stand for crystallization (room temperature, about 25°C), suction filter, and vacuum dry ( 50°C, vacuum degree Figure 5 shown.
Embodiment 3
[0025] Take 10g of the refined product of Alamode, put it into a 1000mL eggplant-shaped flask; add 350ml of a 1:4 DMF / ethanol mixed solvent, reflux at 90°C for 30 minutes, stand for crystallization (in the refrigerator, about -5°C), suction filter, vacuum Dry (50°C, vacuum degree Figure 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com