2,5-furandicarboxamidediamine compound, and preparation method and application thereof
A technology of furandicarboxamide diamine and furandicarboxylic acid diester, which is applied in 2 fields and can solve problems such as difficulty in large-scale production and complex synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 2, the preparation of dimethyl furandicarboxylate:
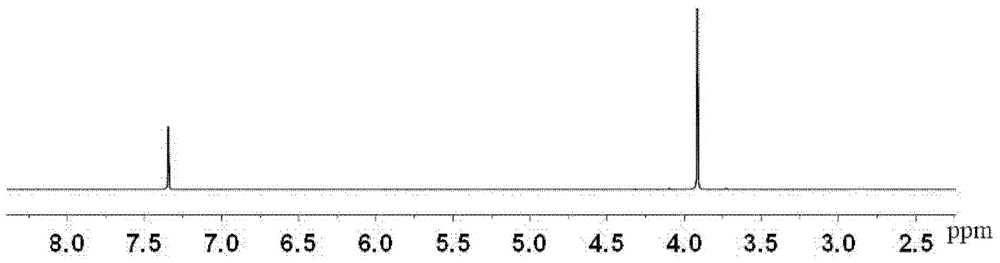
[0065] Mix 100g of 2,5-furandicarboxylic acid, 100g of methanol, and 10g of 98wt% concentrated sulfuric acid evenly. After reacting at 65°C for 12 hours, let it stand still, remove methanol by rotary evaporation under reduced pressure, wash with water and dry to obtain 2,5- Dimethyl furandicarboxylate, the yield was 92%. 1 H-NMR such as figure 1 Shown, CH on the furan ring, 2H, δ (7.34); methyl CH 3 , 6H, δ (3.92).
[0066] 2, the preparation of 5-furan bis(carboxamide ethylamine):
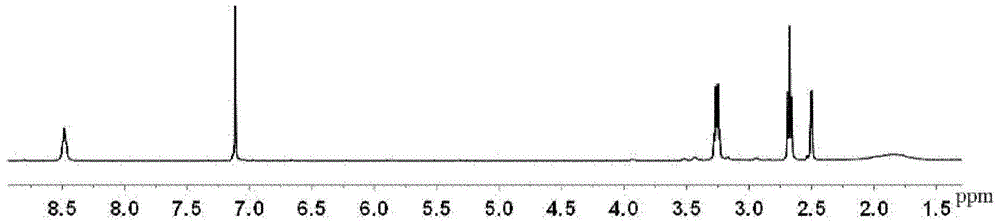
[0067] Dissolve 100 g of dimethyl 2,5-furandicarboxylate prepared according to the above method in 100 g of methanol, add 300 g of ethylenediamine, react at 30°C for 24 hours, distill off excess ethylenediamine and methanol under reduced pressure, and dry to obtain 2,5 -furan bis(formamide ethylamine), structural formula such as IV. 1 H NMR as figure 2 , CH on the furan ring, 2H, δ(7.11); NH, 2H, δ(8.48) in the amide; CH 2 , 4...
Embodiment 2
[0072] 2, the preparation of 5-diethyl furandicarboxylate:
[0073]Mix 100g of 2,5-furandicarboxylic acid, 200g of ethanol, and 3g of p-toluenesulfonic acid evenly, react at 70°C for 18 hours, let it stand, remove ethanol by rotary evaporation under reduced pressure, wash with water and dry to obtain 2,5-furan Diethyl dicarboxylate, yield 90%.
[0074] 2, the preparation of 5-furan bis(carboxamide butylamine):
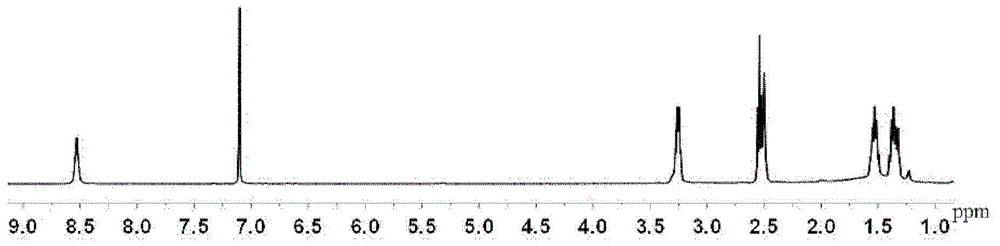
[0075] Dissolve 100 g of diethyl 2,5-furandicarboxylate prepared according to the above method in 200 g of ethanol, add 400 g of butanediamine, react at 40°C for 20 hours, remove excess butanediamine and ethanol by rotary evaporation under reduced pressure, and dry to obtain 2 , 5-furan bis(carboxamide butylamine), structural formula such as V. 1 H NMR as image 3 , CH, 2H, δ(7.10) on the furan ring; NH, 2H, δ(8.53) amides; CH 2 , 4H, δ(3.26); CH 2 , 4H, δ(2.54); CH 2 , 4H, δ(1.53); CH 2 , 4H, δ(1.36); terminal amino NH 2 , 4H, δ(1.65), the yield was 83%. ...
Embodiment 3
[0080] 2, the preparation of 5-dipropyl furandicarboxylate:
[0081] 100g of 2,5-furandicarboxylic acid, 300g of propanol, and 2g of crystalline aluminum trichloride were reacted at 75°C for 20 hours, left to stand, and the propanol was removed by vacuum rotary evaporation, washed with water and dried to obtain 2,5-furan Dipropyl diformate, yield 93%.
[0082] 2, the preparation of 5-furan two (carboxamide hexylamine):
[0083] Dissolve 100 g of dipropyl 2,5-furandicarboxylate prepared by the above method in 400 g of ethanol, add 600 g of hexamethylenediamine, react at 60°C for 24 hours, remove excess hexamethylenediamine and ethanol by rotary evaporation under reduced pressure, and dry to obtain 2 , 5-furan bis(carboxamide hexylamine), structural formula such as V. 1 H NMR as Figure 4 , CH on the furan ring, 2H, δ(7.10); NH, 2H, δ(7.83) in the amide; CH 2 , 4H, δ(3.22); CH 2 , 4H, δ(2.74); CH 2 , 8H, δ(1.53); CH 2 , 8H, δ(1.30); 80% yield.
[0084]
[0085] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile modulus | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Bending strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com