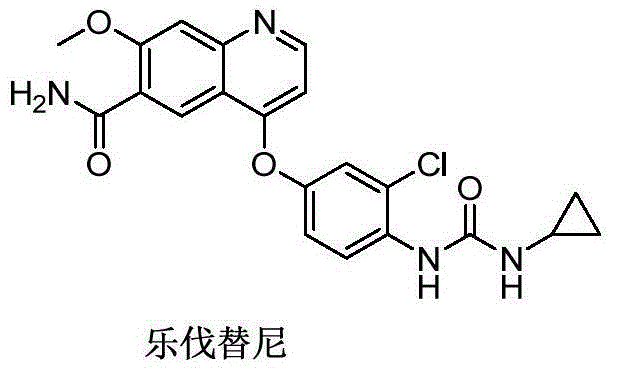
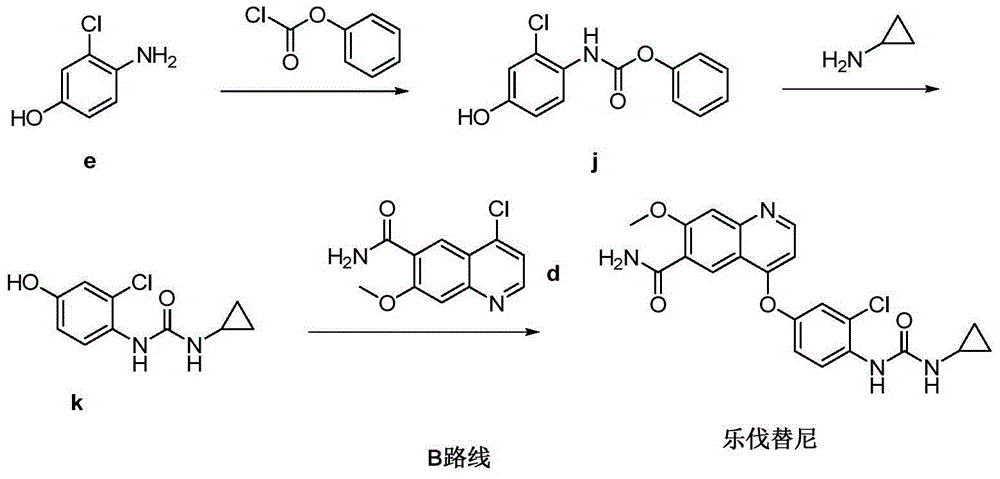
Preparation method of lenvatinib
A technology of lenvatinib and compounds, applied in the field of drug synthesis, can solve the problems of many reaction steps, long reaction time, and difficult post-processing, and achieve the effects of less difficult-to-remove impurities, convenient research, and convenient quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of compound m
[0033]
[0034] At 25°C, N,N'-carbonyldiimidazole (1g, 6.1mmol) was stirred and dissolved in dichloromethane (10ml), and cyclopropylamine (0.35g, 6.1mmol) was slowly added dropwise, and the reaction was monitored by TLC. The reaction solution was washed with water (5ml×2), the organic phase was dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain compound m (0.77g) with a yield of 82.5%.
[0035] ESI-MS[M+H] + :152.0821.
[0036] 1 H NMR (300MHz, DMSO-d6): δ8.5139(s,1H),8.1999(s,1H),7.6316(s,1H),7.0034(s,1H),2.7471(m,1H),0.7363(m ,2H),0.6111(m,2H).
[0037] 13 C NMR (75 MHz, DMSO-d6): δ 149.60, 135.81, 129.38, 116.52, 23.12, 5.67.
Embodiment 2
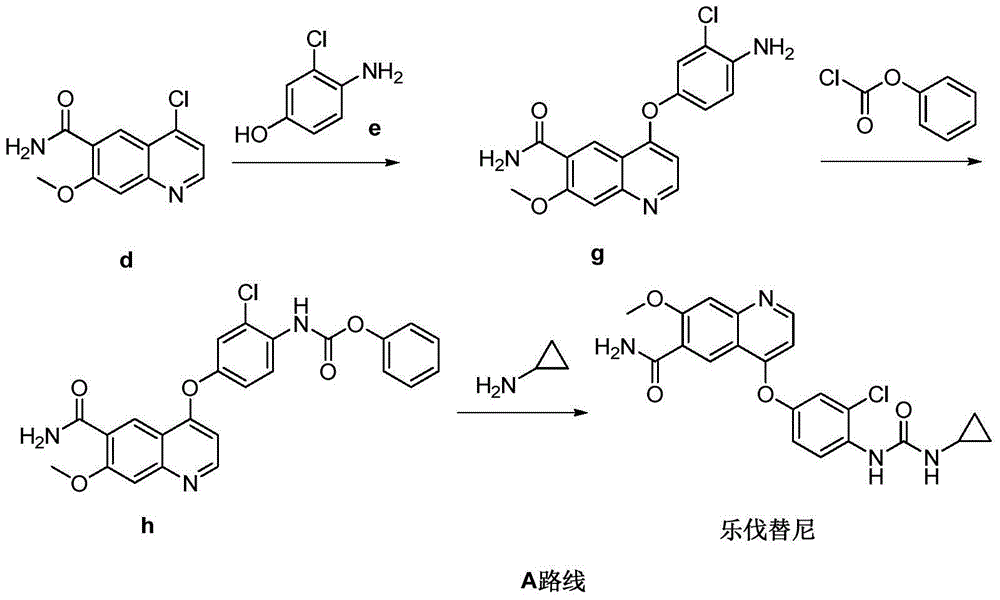
[0038] Embodiment 2: the preparation of lenvatinib
[0039]
[0040] Add 4-(4-amino-3-chlorophenoxy)-7-methoxyquinoline-6-carboxamide (compound g) (1 g, 2.9 mmol) and compound m (0.44 g, 2.9 mmol) into the reaction vessel mmol), then add DMF (10ml), stir and dissolve at 25°C, add N,N'-diisopropylethylamine (0.75g, 5.8mmol) after complete dissolution, the reaction solution is slowly heated to 85°C, monitored by TLC The reaction is over. Add water (50ml) and stir, filter, wash the filter cake with water, and dry to obtain Delvatinib (1.1g), the yield is 88.6%. Purity: 98.7%.
[0041] ESI-MS[M+H] + :427.1144.
[0042] 1 H NMR (300MHz, DMSO-d6): δ8.6755(s, 2H), 8.2794(d, J=9.1Hz, 1H), 7.9722(s, 1H), 7.8388(s, 1H), 7.7132(s, 1H ), 7.5209(m, 1H), 7.4876(d, J=2.6Hz, 1H), 7.2442(dd, J=2.6, 9.1Hz, 1H), 7.1869(d, J=2.6Hz, 1H), 6.5360(d , J=5.2Hz, 1H), 4.0376(s, 3H), 2.5736(m, 1H), 0.6723(m, 2H), 0.4370(m, 2H).
[0043] 13 C NMR(75MHz,DMSO-d6):δ165.71,161.49,158.00,155.41,153...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com