Application of composition of ginsenoside C-K and lamotrigine in preparation of anti-epilepsy medicines
An anti-epileptic drug and ginsenoside technology are applied in the field of medicine to achieve the effects of enhancing effect and curative effect, reducing dosage and reducing adverse drug reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
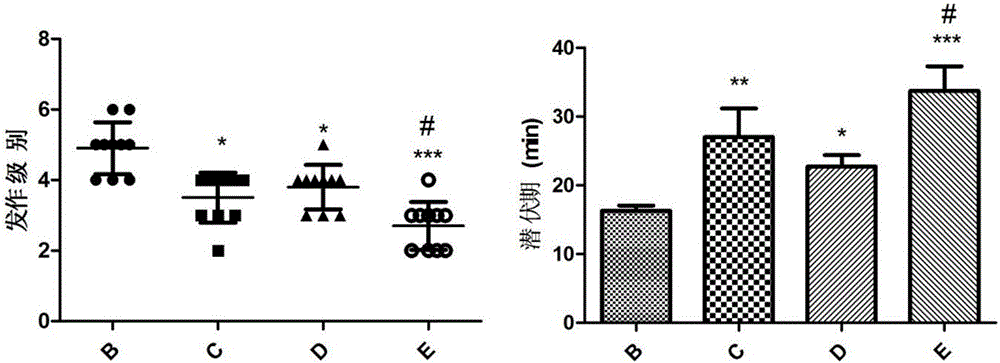
[0014] Example 1 The composition of ginsenoside C-K and lamotrigine interferes with the acute epilepsy model induced by lithium chloride-pilocarpine
[0015] 1. Experimental method
[0016] 1.1 Dose Design and Grouping
[0017] The experiment was divided into normal control group, epilepsy model group, lamotrigine group (20mg / kg), ginsenoside C-K group (160mg / kg), ginsenoside C-K (160mg / kg) combined with lamotrigine group (20mg / kg )Group. (See Table 1).
[0018] Table 1 Experimental grouping and dose design
[0019]
[0020] 1.2 Experimental steps
[0021] 50 SPF grade healthy male SD rats (body weight 180-220g) were randomly divided into normal control group (group A), epilepsy model group (group B), lamotrigine group (group C), ginsenoside C-K group ( D group), ginsenoside C-K+lamotrigine group (E group), 10 rats in each group. Lamotrigine or ginsenosides C-K were prepared with 0.5% CMC-Na to the corresponding concentration according to the dose before the daily exp...
Embodiment 2
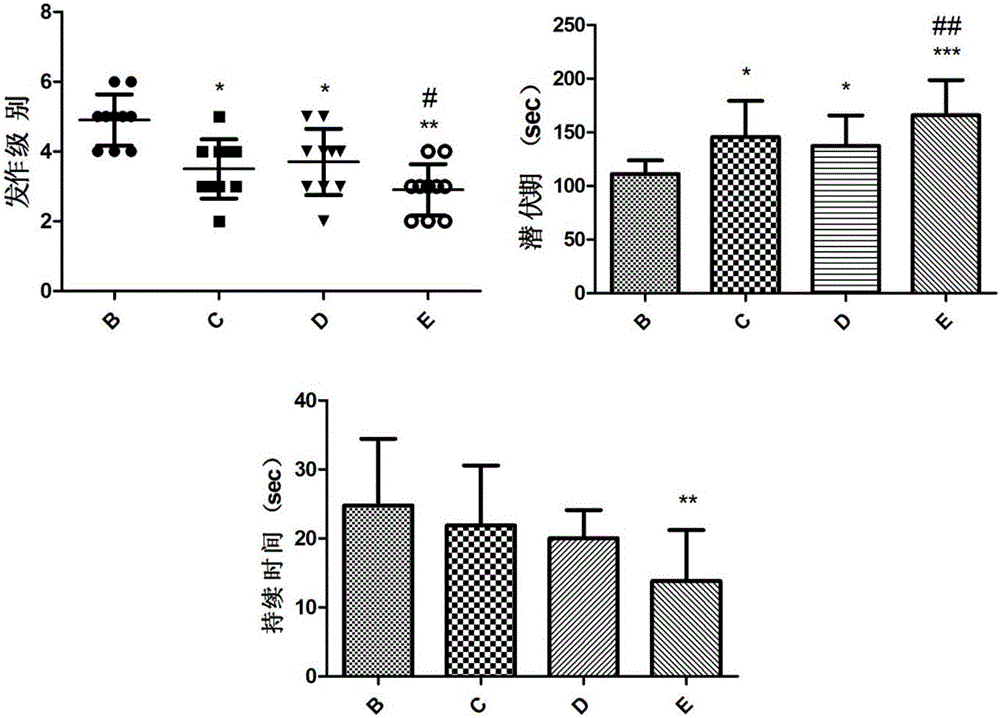
[0031] Example 2 The composition of ginsenoside C-K and lamotrigine interferes with the acute epilepsy model induced by pentylenetetrazol
[0032] 1. Experimental method
[0033] 1.1 Dose Design and Grouping
[0034] The experiment was divided into normal control group, epilepsy model group, lamotrigine group (20mg / kg), ginsenoside C-K group (160mg / kg), ginsenoside C-K (160mg / kg) combined with lamotrigine group (20mg / kg )Group. (See Table 3).
[0035] Table 3 Experimental grouping and dose design
[0036]
[0037] 1.2 Experimental steps
[0038] 50 SPF grade healthy male SD rats (body weight 180-220g) were randomly divided into normal control group (group A), epilepsy model group (group B), lamotrigine group (group C), ginsenoside C-K group ( D group), ginsenoside C-K+lamotrigine group (E group), 10 rats in each group. Lamotrigine or ginsenosides C-K were prepared with 0.5% CMC-Na to the corresponding concentration according to the dose before the daily experiment, an...
Embodiment 3
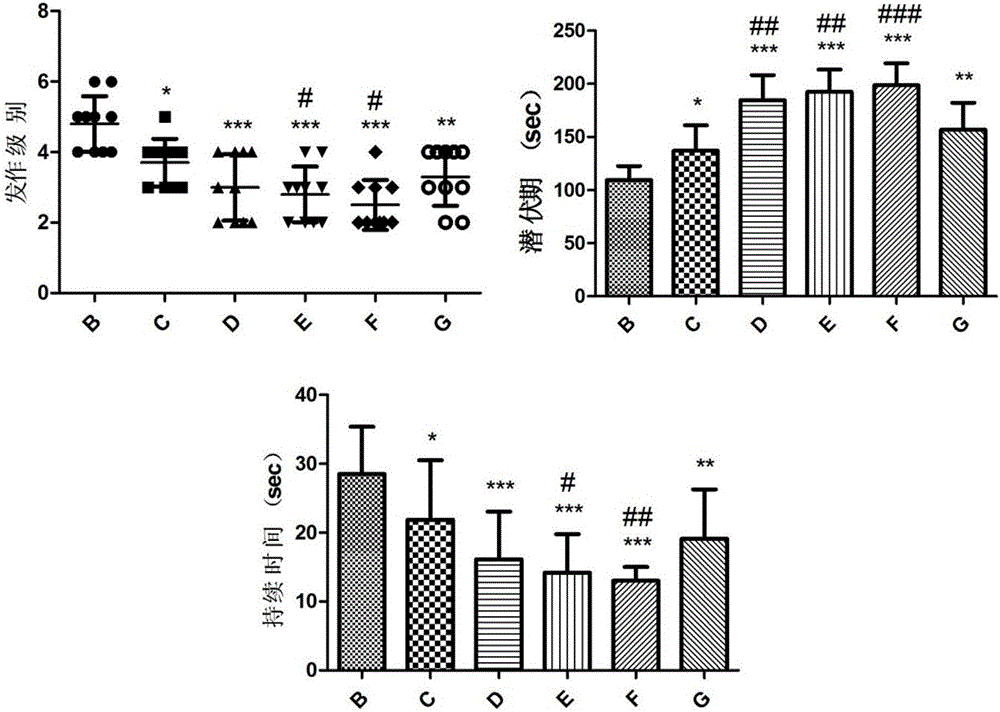
[0048] Example 3 The composition of ginsenoside C-K and lamotrigine in different mass ratios interferes with the acute epilepsy model induced by pentylenetetrazol
[0049] 1. Experimental method
[0050] 1.1 Dose Design and Grouping
[0051] The experiment was divided into normal control group, epilepsy model group and compound intervention group. (See Table 4).
[0052] Table 4 group administration
[0053]
[0054] 1.2 Experimental steps
[0055] 70 SPF grade healthy male SD rats (body weight 180-220g), were randomly divided into normal control group (group A), epilepsy model group (group B), composition intervention 1, 2, 3, 4, 5 groups (group C) , D, E, F, G groups), 10 rats in each group. Before the daily experiment, lamotrigine, ginsenosides C-K and the composition were formulated with 0.5% CMC-Na according to the dose to the corresponding concentration, and they were prepared and used immediately. The body weight of the rats was weighed before administration ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com