Methods of Fluorinating Compounds
A fluorination, cyanide technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as poor selectivity and reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
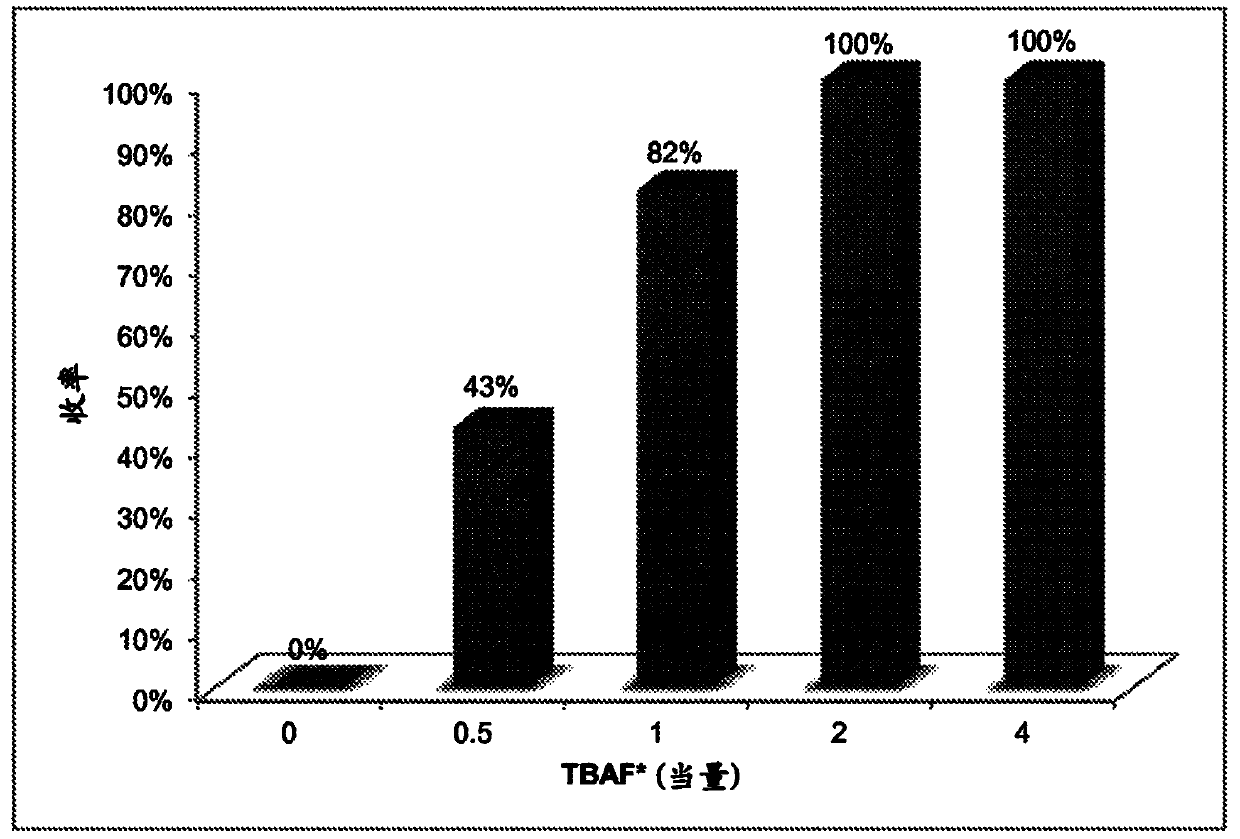
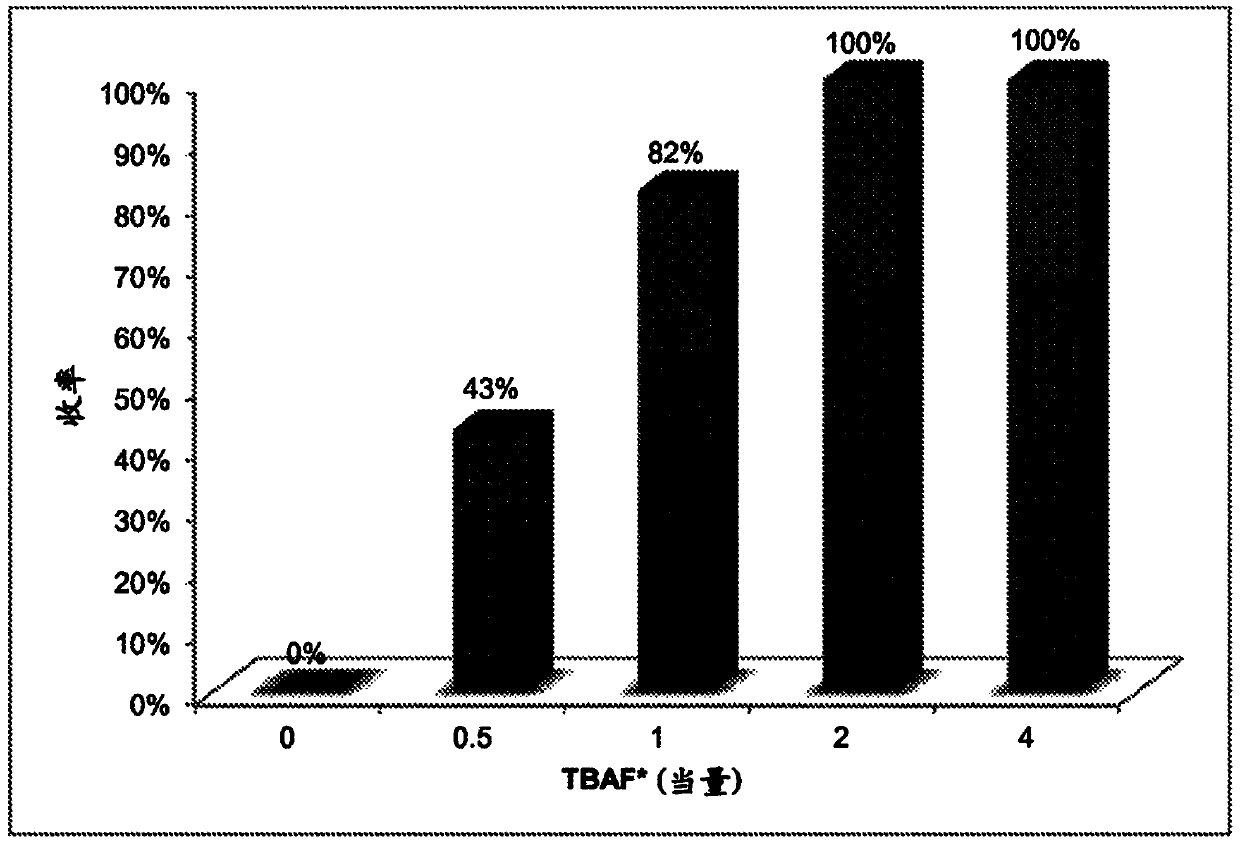
[0097] The following examples are set forth to illustrate methods, compositions, and results in accordance with the disclosed subject matter. These examples are not intended to cover all aspects of the subject matter disclosed herein, but rather illustrate representative methods, compositions, and results. These examples do not exclude equivalents and modifications of the present invention, which are obvious to those skilled in the art.
[0098] Efforts have been made to ensure accuracy with respect to numbers (eg, amounts, temperature, etc.), but some errors and deviations should be accounted for. Unless indicated otherwise, parts are parts by weight, temperature is in degrees Celsius (° C.) or is at ambient temperature, and pressure is at or near atmospheric. There are many variations and combinations of reaction conditions, eg, component concentrations, temperatures, pressures, and other reaction ranges and conditions that can be used to optimize product purity and yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com