Aprindine hydrochloride medicinal composition and medicinal use thereof
A technology of aplindine hydrochloride and its composition, which is applied in the field of pharmaceutical composition of aplindine hydrochloride, can solve the problems that have not yet been reported on the sedation and tranquilization of aplindine hydrochloride, and achieve outstanding substantive features, sedative Strong calming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
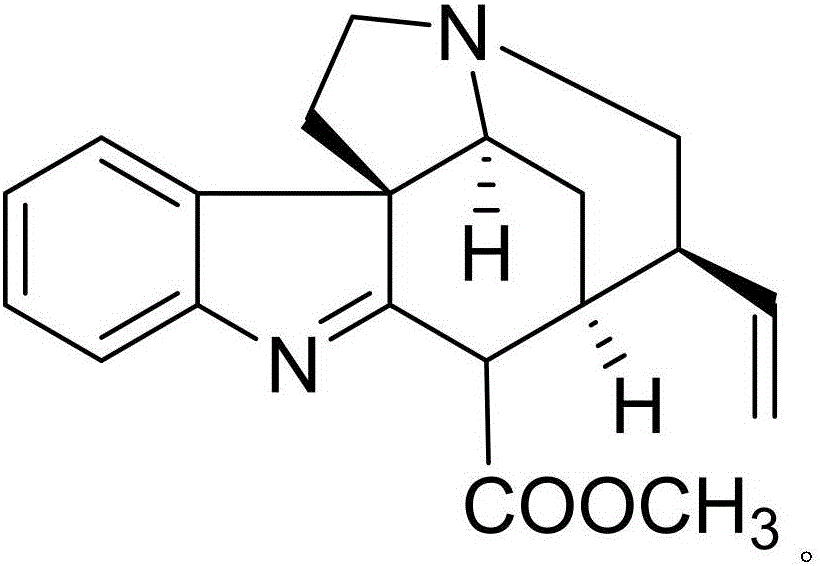
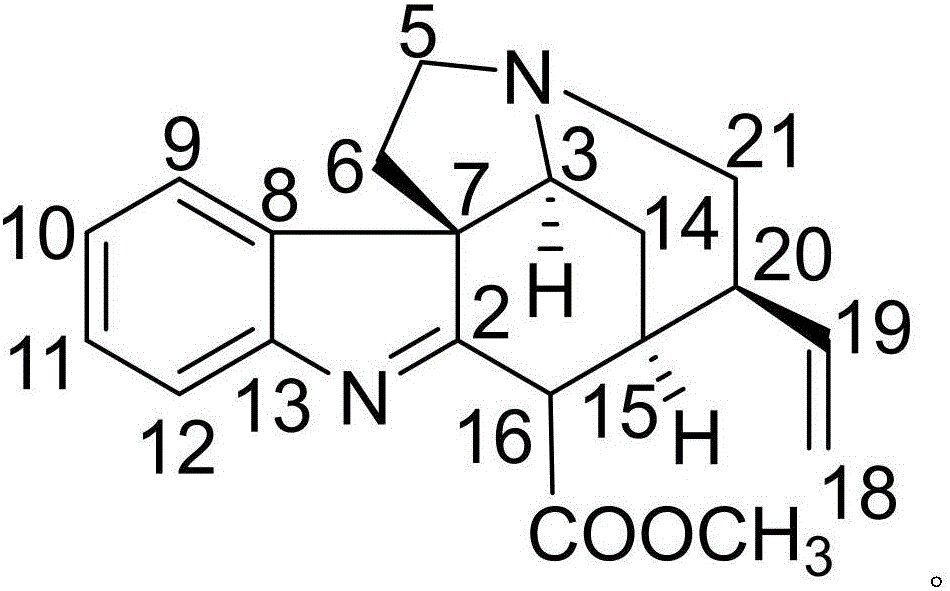
[0021] Example 1: Compound (I) Separation Preparation and Structure Confirmation
[0022] Sources of reagents: ethanol, petroleum ether, ethyl acetate, n-butanol, and dichloromethane were of analytical grade, purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Methanol, of analytical grade, were purchased from Jiangsu Hanbang Chemical Reagent Co., Ltd.
[0023] Separation method: (a) Grind the dried tuber root (2kg) of Pseudostellariae Pseudostellariae, extract with 80% ethanol under heat reflux (15L×3 times), combine the extracts, concentrate until there is no alcohol smell (3L), and then use petroleum ether (3L ×3 times), ethyl acetate (3L×3 times) and water-saturated n-butanol (3L×3 times) were extracted to obtain petroleum ether extract, ethyl acetate extract and n-butanol extract respectively; (b) In the step (a), the n-butanol extract is removed with a D101 type macroporous resin, and first eluted with 10% ethanol for 8 column volumes, then with 70% ethanol for 1...
Embodiment 2
[0026] Embodiment 2: pharmacological action
[0027] 1. Materials and methods
[0028] 1.1 Animals
[0029] Kunming mice, weighing 18-25 g, were bred and provided by the Laboratory Animal Laboratory of China Pharmaceutical University.
[0030]1.2 Reagents and samples
[0031] Apulin hydrochloride was purchased from China National Institute for the Control of Pharmaceutical and Biological Products. Compound (I) is self-made, and the preparation method is shown in Example 1.
[0032] 1.3 Mouse grouping and model preparation
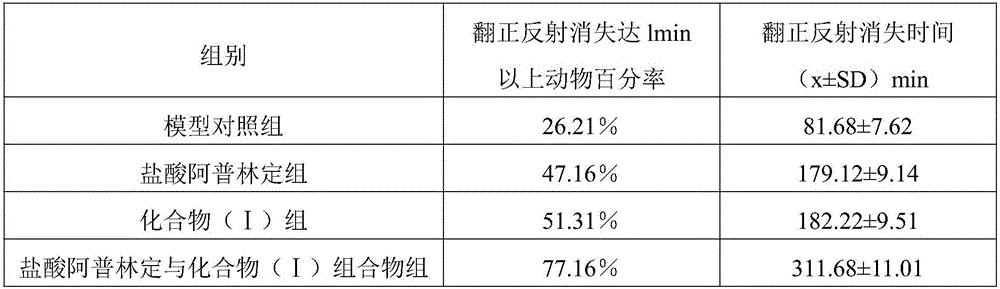
[0033] The mice were randomly divided into 4 groups, 12 in each group, respectively the model control group, the aplinidine hydrochloride group (40 mg·kg -1 ), compound (I) group (40mg·kg -1 ), aplindine hydrochloride and compound (I) composition group [20mg·kg -1 Aplindine hydrochloride+20mg·kg -1 Compound (I)], orally administered by gavage.
[0034] 1.4 Pentobarbital Sodium Subthreshold Hypnotic Dose Determination
[0035] 1 hour after intragas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com