Preparation method of azilsartan impurity a and b
A technology of impurities and intermediates, applied in the field of preparation of Azilsartan impurities A and B, achieves the effects of high yield, avoiding the formation of impurities and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
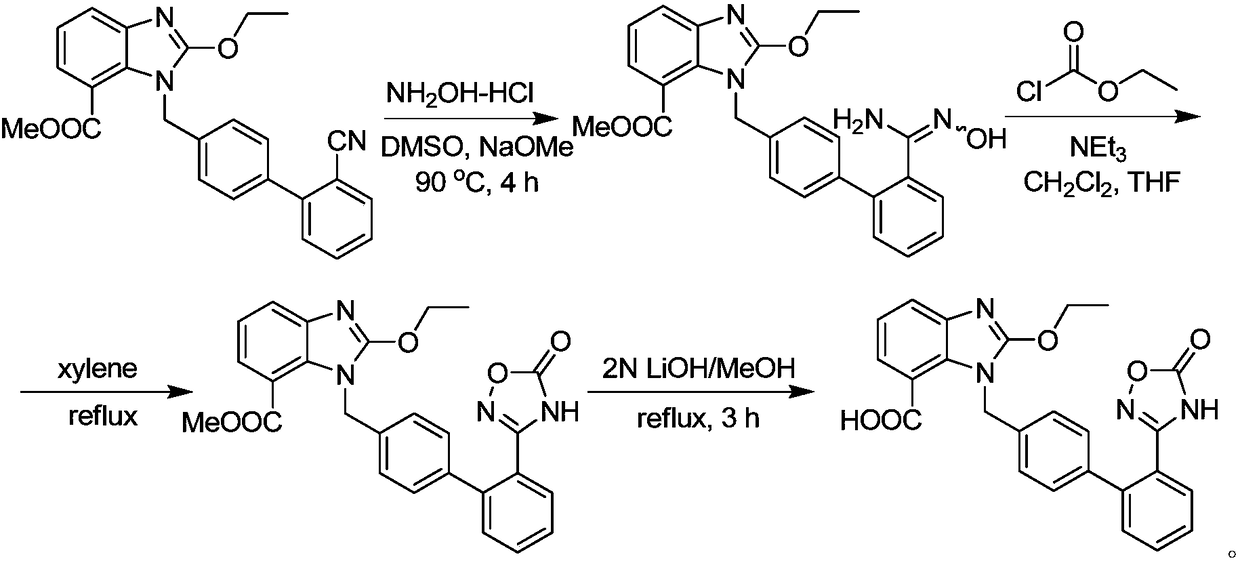
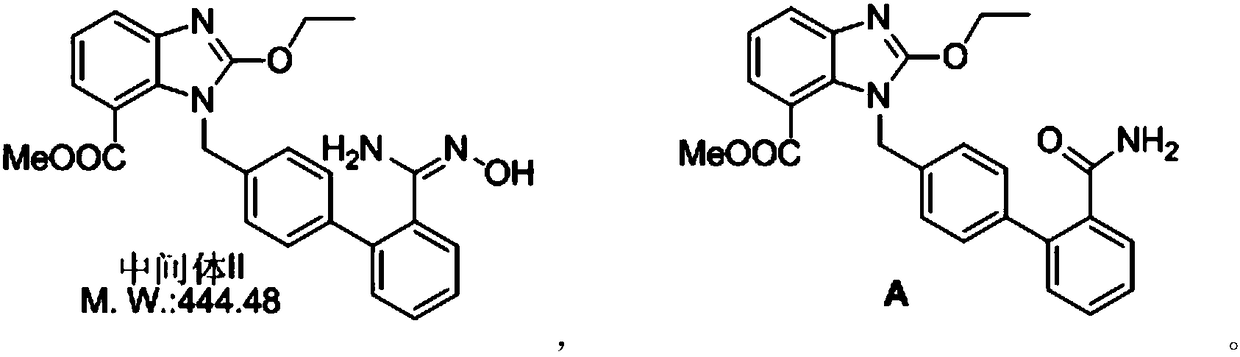
[0025] Add 10.0g (22.5mmol) of intermediate II to 100ml of 1,4-dioxane, heat to reflux, slowly add 3.84g (23.7mmol, 1.05eq) of carbonyldiimidazole in 50ml of 1,4-dioxane Oxycycline solution, after the dropwise addition, reflux reaction for 5 hours; stop the reaction, remove the solvent by distillation under reduced pressure; dissolve the residue in ethyl acetate, add water, under stirring, adjust the pH to 3 with HCl, let stand to separate, remove Aqueous phase; adding water to wash, separating and removing the water phase, washing the organic phase with saturated brine, removing the water phase, cooling and crystallizing the organic phase with an ice-water bath for 1 hour, filtering, and washing with a small amount of ethyl acetate to obtain impurity A, the product 85% rate
[0026] The product has passed the identification of mass spectrometry and NMR:
[0027] ESI-MS: 430.2 (M+1), 452.2 (M+Na).
[0028] 1H-NMR (600MHz, DMSO-d 6 )δ: 1.42(3H,t,J=6Hz),3.71(3H,s),4.62(2H,q,J...
Embodiment 2
[0031] Add 10.0g (22.5mmol) of intermediate II to 100ml of tetrahydrofuran, heat to reflux, slowly add dropwise 4.01g (24.7mmol, 1.1eq) carbonyldiimidazole in 50ml of tetrahydrofuran solution, after the dropwise addition is completed, reflux for 5 hours Stop the reaction, remove the solvent by distillation under reduced pressure; dissolve the residue in ethyl acetate, add water, under stirring, adjust the pH to 5 with HCl, leave to separate layers, and remove the water phase; add water to wash, separate the liquid to remove the water phase, organic The phase was washed with saturated brine, and the water phase was removed. The organic phase was cooled and crystallized in an ice-water bath for 1 hour, filtered, and washed with a small amount of ethyl acetate to obtain impurity A.
Embodiment 3
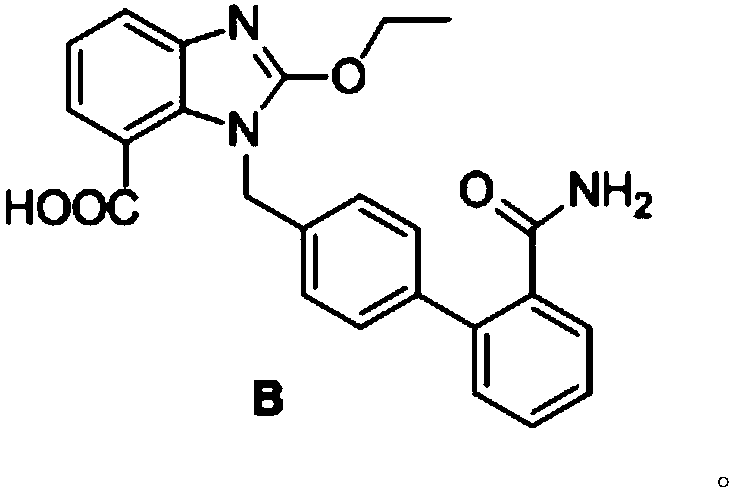
[0033] Add 4.3g of impurity A to 100mL of water, add dropwise 2N LiOH aqueous solution until the solid dissolves, then add 1mL, heat up to 75±3°C, and react for 1 hour;
[0034] Stop heating, add absolute ethanol, cool down to below 30°C, adjust the pH to 2.3±0.1 with 2M-HCl, and slowly precipitate a white insoluble solid; filter, wash with purified water, wash with water three times, each 10mL, dry to obtain impurity B , as a white powdery solid with a yield of 99%.
[0035] The product has passed the identification of mass spectrometry and NMR:
[0036] ESI-MS: 416.2 (M+1), 438.2 (M+Na).
[0037] 1H-NMR (600MHz, DMSO-d 6 )δ: 1.41 (3H, t, J = 6, 12Hz), 4.60 (2H, q, J = 6, 12, 6Hz), 5.68 (2H, s), 7.04 (2H, q, J = 6, 12, 12Hz), 7.18(1H,t,J=6,12Hz),7.26(1H,s),7.30-7.38(4H,m),7.41-7.47(2H,m),7.56(1H,d,J=6Hz ), 7.64 (1H, s), 7.68 (1H, d, J=6Hz), 13.13 (1H, br).
[0038] 13C-NMR (150MHz, DMSO-d 6 )δ: 14.5, 46.3, 66.6, 116.8, 120.7, 121.4, 123.5, 126.3, 127.0, 127.5, 128.6, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com