Anti-fibrosis drug pirfenidone crystal forms and preparation method thereof
A technology of pirfenidone and anti-fibrosis, which is applied in the field of anti-fibrosis drug pirfenidone crystal form and its preparation, can solve the problems of no pirfenidone crystal form, etc., and achieve stable chemical and physical properties, crystal particles Uniform, easy-to-manufacture effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
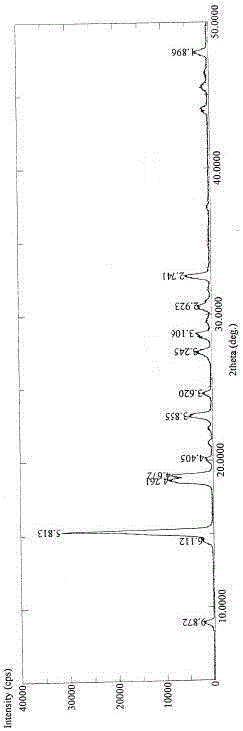
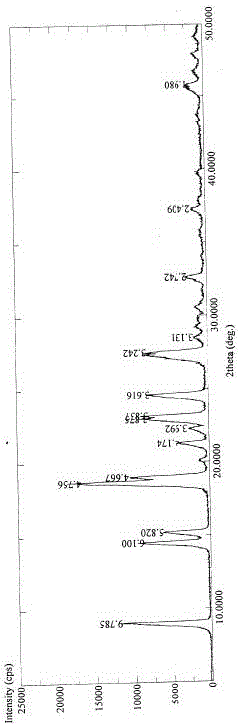
[0020] Put 10g of pirfenidone in a 100mL two-necked flask, add 60mL of ethanol solution with a volume fraction of 10%, heat and stir in an oil bath until the raw material is completely dissolved, stop heating after the raw material is completely dissolved, and gradually cool down to 0-5°C Set aside for two hours, filter, and dry under normal pressure at 50° C. for 12 hours to obtain pirfenidone crystal form II powder.
Embodiment 2
[0022] Put 10g of pirfenidone in a 100mL two-necked flask, add 40mL of absolute ethanol, heat and stir in an oil bath until the raw materials are dissolved, stop heating after the raw materials are completely dissolved, gradually cool down to 0-5°C and let stand for two hours, filter, Drying at 50° C. for 12 hours under normal pressure with forced air to obtain the powder of pirfenidone crystal form III.
Embodiment 3
[0024] Put 10g of pirfenidone in a 500mL two-necked flask, add 50mL of ethyl acetate, stir at room temperature until the raw material is completely dissolved, gradually add 300mL of n-heptane dropwise under stirring, and keep warm at 0-5°C for 8 hours after the dropwise addition Filtrate, and dry at 50° C. under normal pressure for 12 hours to obtain the powder of pirfenidone crystal form III.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com