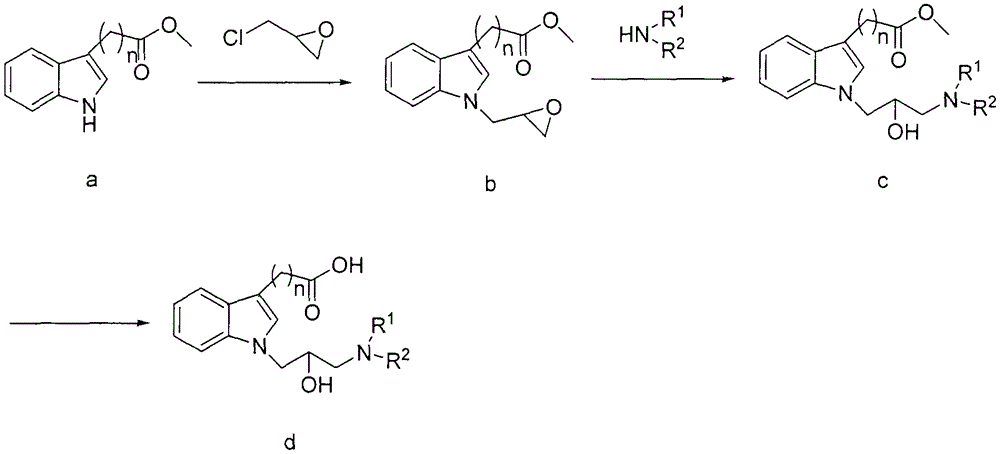
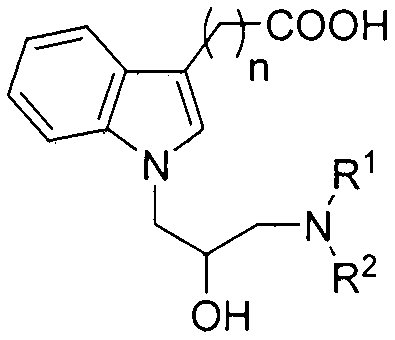
N-substituted indole carboxylic acid derivative and its preparation method and medical use
A technology of indole carboxylic acid and its derivatives, which is applied in the fields of drug combination, antineoplastic drugs, organic chemistry, etc., and can solve problems such as poor selectivity and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 1-(oxirane-2-ylmethyl)-1H-indole-3-carboxylic acid methyl ester (b-1):
[0031] Add methyl 3-indolecarboxylate (8.25g, 0.047mol) and 50ml DMSO into a 250ml three-necked flask, stir to dissolve, add 60% sodium hydrogen (2.84 g, 0.071mol), stirred at room temperature for 30 minutes. A solution of epichlorohydrin (13.07 g, 0.141 mol) in DMSO (30 ml) was slowly added dropwise to the reaction flask under an ice bath (internal temperature 0-10° C.). After dropping, the temperature was naturally raised to room temperature and the reaction was stirred for 12 hours. Add 250ml of water for dilution, extract with ethyl acetate (120ml×3), combine the organic phases, wash with water and saturated brine, concentrate the organic phase and perform column chromatography (eluent: petroleum ether: ethyl acetate 8:1) , to obtain 3.65 g of brown oil, yield 33.5%.
[0032] 1 H-NMR (300MHz, CDCl 3 ), δ (ppm): 8.11-7.17 (m, 5H), 4.36 (dd, J 1 =2.76Hz,J 2 =15.21Hz, 1H), 4.05...
Embodiment 2
[0035] Synthesis of 2-(1-(oxirane-2-ylmethyl)-1H-indol-3-yl)methyl acetate (b-2):
[0036] The target compound was prepared by a method similar to Example 1 as a yellow oil with a yield of 33.6%.
[0037] 1 H-NMR (300MHz, CDCl 3 ), δ (ppm): 7.68-7.16 (m, 5H), 4.36 (dd, J 1 =2.82Hz,J 2 =15.27Hz, 1H), 4.08(dd, J 1 =5.37Hz,J 2 =15.24Hz, 1H), 3.84(s, 2H), 3.76(s, 3H), 3.25(m, 1H), 2.80(t, J=4.32Hz, 1H), 2.48(dd, J 1 =2.40Hz,J 2 =4.26Hz, 1H)
[0038] MS(ESI)m / z: 246[M+H] +
Embodiment 3
[0040] Synthesis of 3-(1-(oxirane-2-ylmethyl)-1H-indol-3-yl)propionic acid methyl ester (b-3):
[0041] The target compound was prepared by a method similar to Example 1 as a white translucent oil with a yield of 19.3%.
[0042] 1 H-NMR (300MHz, CDCl 3 ), δ (ppm): 7.58-6.88 (m, 5H), 4.30 (dd, J 1 =3.21Hz,J 2 =15.24Hz, 1H), 4.07(dd, J 1 =5.28Hz,J 2 =15.27Hz, 1H), 3.64(s, 3H), 3.20(m, 1H), 3.10(t, J=7.11Hz, 2H), 2.72-2.78(m, 3H), 2.40(dd, J 1 =2.55Hz,J 2 =4.71Hz, 1H)
[0043] MS(ESI)m / z: 260[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com